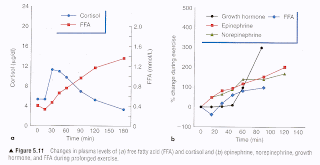
The figure below (click to enlarge) is from the outstanding book Physiology of sport and exercise, by Jack H. Wilmore, David L. Costill, and W. Larry Kenney. If you are serious about endurance or resistance exercise, or want to have a deeper understanding of exercise physiology beyond what one can get in popular exercise books, this book should be in your personal and/or institutional library. It is one of the most comprehensive textbooks on exercise physiology around. The full reference to the book is at the end of this post.
The hormonal and free fatty acid responses shown on the two graphs are to relatively intense exercise combining aerobic and anaerobic components. Something like competitive cross-country running in an area with hills would lead to that type of response. As you can see, cortisol spikes at the beginning, combining forces with adrenaline and noradrenaline (a.k.a. epinephrine and norepinephrine) to quickly increase circulating free fatty acid levels. Then free fatty acid levels are maintained elevated by adrenaline, noradrenaline, and growth hormone. As you can see from the graphs, free fatty acid levels are initially pulled up by cortisol, and then are very strongly correlated with adrenaline and noradrenaline. Those free fatty acids feed muscle, and also lead to the production of ketones, which provide extra fuel for muscle tissue.
Growth hormone stays flat for about 40 minutes, after which it goes up steeply. At around the 90-minute mark, it reaches a level that is quite high; 300 percent higher than it was prior to the exercise session. Natural elevation of circulating growth hormone through intense exercise, intermittent fasting, and restful sleep, leads to a number of health benefits. It helps burn abdominal fat, often hours after the exercise session, and helps builds muscle (in conjunction with other hormones, such as testosterone). It appears to increase insulin sensitivity in the long run. Maybe natural elevation of circulating growth hormone is one of the “secrets” of people like Bob Delmonteque, who is probably the fittest octogenarian in the world today.
Aerobic activities normally do not elevate growth hormone levels, even though they are healthy, unless they lead to a significant degree of glycogen depletion. Glycogen is stored in the liver and muscle, with muscle storing about 5 times more than the liver (about 500 g in adults). Once those reserves go down significantly during exercise, it seems that growth hormone is recruited to ramp up fat catabolism and facilitate other metabolic processes. Walking for an hour, even if briskly, is good for fat burning, but generates only a small growth hormone elevation. Including a few all-out sprints into that walk can help significantly increase growth hormone secretion.
Having said that, it is not really clear whether growth hormone elevation is a response to glycogen depletion, or whether both happen together in response to another stimulus or related metabolic process. There are other factors that come into play as well. For example, circulating growth hormone increase is moderated by sex hormone (e.g., testosterone, estrogen) secretion, thus larger growth hormone increases in response to exercise are observed in older men than in older women. (Testosterone declines more slowly with age in men than estrogen does in women.) Also, growth hormone increase seems to be correlated with an increase in circulating ketones.
Heavy resistance exercise seems to lead to a higher growth hormone elevation per unit of time than endurance exercise. That is, an intense resistance training session lasting only 30 minutes can lead to an acute circulating growth hormone response, similar to that shown on the figure. The key seems to be reaching the point during the exercise where muscle glycogen stores are significantly depleted. Many people who weight-train achieve this regularly by combining a reasonable number of sets (e.g., 6-12), with repetitions in the muscle hypertrophy range (again, 6-12); and progressive overload, whereby resistance is increased incrementally every session.
Progressive overload is needed because glycogen reserves are themselves increased in response to training, so one has to increase resistance every session to keep up with those increases. This goes on only up to a point, a point of saturation, usually reached by elite athletes. Glycogen is the primary fuel for anaerobic exercise; fat is used as fuel in the recovery period between sets, and after the exercise is over. Glycogen is expended proportionally to the number of calories used in the anaerobic effort. Calories are expended proportionally to the total amount of weight moved around, and are also a function of the movements performed (moving a certain weight 1 feet spends less energy than moving it 3 feet). By the way, not much glycogen is depleted in a 30-minute session. The total caloric expenditure will probably be around 250 calories above the basal metabolic rate, which will require about 63 g of glycogen.
Many sensations are associated with reaching the glycogen depletion level required for an acute growth hormone response during heavy anaerobic exercise. Often light to severe nausea is experienced. Many people report a “funny” feeling, which is unmistakable to them, but very difficult to describe. In some people the “funny” feeling is followed, after even more exertion, by a progressively strong sensation of “pins and needles”, which, unlike that associated with a heart attack, comes slowly and also goes away slowly with rest. Some people feel lightheaded as well.
It seems that the optimal point is reached immediately before the above sensations become bothersome; perhaps at the onset of the “funny” feeling. My personal impression is that the level at which one experiences the “pins and needles” sensation should be avoided, because that is a point where your body is about to “force” you to stop exercising. (Note: I am not a bodybuilder; see “Interesting links” for more extensive resources on the subject.) Besides, go to that point or beyond and significant muscle catabolism may occur, because the body prioritizes glycogen reserves over muscle protein. It will break that protein down to produce glucose via gluconeogenesis to feed muscle glycogenesis.
That the body prioritizes muscle glycogen reserves over muscle protein is surprising to many, but makes evolutionary sense. In our evolutionary past, there were no selection pressures on humans to win bodybuilding tournaments. For our hominid ancestors, it was more important to have the glycogen tank at least half-full than to have some extra muscle protein. Without glycogen, the violent muscle contractions needed for a “fight or flight” response to an animal attack simply cannot happen. And large predators (e.g., a bear) would not feel intimated by big human muscles alone; it would be the human’s response using those muscles that would result in survival or death.
Overall, selection pressures probably favored functional strength combined with endurance, leading to body types similar to those of the hunter-gatherers shown on this post.
Even though the growth hormone response to exercise can be steep, the highest natural growth hormone spike seems to be the one that occurs at night, during deep sleep.
Exercising hard pays off, but only if one sleeps well.
Reference:
Wilmore, J.H., Costill, D.L., & Kenney, W.L. (2007). Physiology of sport and exercise. Champaign, IL: Human Kinetics.
Sunday, May 30, 2010
Friday, May 28, 2010
62-yr-old Woman with Hypertention, Ventricular Hypertrophy and Congestive Heart Failure
One of the considerations with congestive heart failure is the need for fluid restriction and the patient will need to work her doctor to be able understand how much she should be getting daily.
Sodium restriction is important for bringing down the blood pressure. In the case of this woman, I would employ a DASH diet to bring down her blood pressure with emphasis on plenty of fruits and vegetables as well as dairy products such as yogurt to obtain regular amounts of calcium.
Since being overweight contributes to higher blood pressure, if she is overweight, then the DASH diet should be combined with a weight loss program by restriction of calories.
Regular aerobic exercise can also support healthy blood pressure levels. I'd recommend about 30 minutes three times weekly.
Because of her condition, I'd also recommend supplementation with CoQ10 to support the function of the heart. If she has a low vitamin D status, which is associated with higher blood pressure, then I'd also recommend a vitamin D supplement.
Sodium restriction is important for bringing down the blood pressure. In the case of this woman, I would employ a DASH diet to bring down her blood pressure with emphasis on plenty of fruits and vegetables as well as dairy products such as yogurt to obtain regular amounts of calcium.
Since being overweight contributes to higher blood pressure, if she is overweight, then the DASH diet should be combined with a weight loss program by restriction of calories.
Regular aerobic exercise can also support healthy blood pressure levels. I'd recommend about 30 minutes three times weekly.
Because of her condition, I'd also recommend supplementation with CoQ10 to support the function of the heart. If she has a low vitamin D status, which is associated with higher blood pressure, then I'd also recommend a vitamin D supplement.
Labels:
nutr therap
Thursday, May 27, 2010
Postprandial glucose levels, HbA1c, and arterial stiffness: Compared to glucose, lipids are not even on the radar screen
Postprandial glucose levels are the levels of blood glucose after meals. In Western urban environments, the main contributors to elevated postprandial glucose are foods rich in refined carbohydrates and sugars. While postprandial glucose levels may vary somewhat erratically, they are particularly elevated in the morning after breakfast. The main reason for this is that breakfast, in Western urban environments, is typically very high in refined carbohydrates and sugars.
HbA1c, or glycated hemoglobin, is a measure of average blood glucose over a period of a few months. Blood glucose glycates (i.e., sticks to) hemoglobin, a protein found in red blood cells. Red blood cells are relatively long-lived, lasting approximately 3 months. Thus HbA1c (given in percentages) is a good indicator of average blood glucose levels, if you don’t suffer from anemia or a few other blood abnormalities.
Based on HbA1c, one can then estimate his or her average blood glucose level for the previous 3 months or so before the test, using one of the following equations, depending on whether the measurement is in mg/dl or mmol/l.
Average blood glucose (mg/dl) = 28.7 × HbA1c − 46.7
Average blood glucose (mmol/l) = 1.59 × HbA1c − 2.59
Elevated blood glucose levels cause damage in the body primarily through glycation, which leads to the formation of advanced glycation endproducts (AGEs). Given this, HbA1c can be seen as a proxy for the level of damage done by elevated blood glucose levels to various body tissues. This damage occurs over time; often after many years of high blood glucose levels. It includes kidney damage, neurological damage, cardiovascular damage, and damage to the retina.
Most regular blood exams focus on fasting blood glucose as a measure of glucose metabolism status. Many medical practitioners have as a target a fasting blood glucose level of 125 mg/dl (7 mmol/l) or less, and largely disregard postprandial glucose levels or HbA1c in their management of glucose metabolism. Leiter and colleagues (2005; full reference at the end of this post) showed that this focus on fasting blood glucose is a mistake. They are not alone; many others made this point, including some very knowledgeable bloggers who focus on diabetes (see “Interesting links” section of this blog). Leiter and colleagues (2005) also provided some interesting graphs and figures, including eye-opening correlations between various variables and arterial stiffness. The figure below (click to enlarge) shows the contribution of postprandial glucose to HbA1c.
Note that the lower the HbA1c is in the figure (horizontal axis), the higher is the postprandial glucose contribution to HbA1c. And, the lower the HbA1c, the closer the individuals are to what one could consider having a perfectly normal HbA1c level (around 5 percent). That is, only for individuals whose HbA1c levels are very high, fasting blood glucose levels are relatively reliable measures of the tissue damage done be elevated blood glucose levels.
The table below (click to enlarge) shows P values associated with the impact of various variables (listed on the leftmost column) on arterial stiffness. This measure, arterial stiffness, is strongly associated with an increased risk of cardiovascular events. Look at the middle column showing P values adjusted for age and height. The lower the P value, the more a variable affects arterial stiffness. The variable with the lowest P value by far is 2-hour postprandial blood glucose; the blood glucose levels measured 2 hours after meals.
Fasting glucose levels were reported to be statistically insignificant because of the P = 0.049, in terms of their effect on arterial stiffness, but this P value is actually significant, although barely, at the 0.05 level (95 percent confidence). Interestingly, the following measures are not even on the radar screen, as far as arterial stiffness is concerned: systolic blood pressure, LDL cholesterol, HDL cholesterol, triglycerides, and fasting insulin levels.
What about the lipid hypothesis, and the “bad” LDL cholesterol!? This study is telling us that these are not very relevant for arterial stiffness when we control for the effect of blood glucose measures. Not even fasting insulin levels matters much! Wait, not even HDL!!! A high HDL has been definitely shown to be protective, but when we look at the relative magnitude of various effects, the story is a bit different. A high HDL’s protective effect exists, but it is dwarfed by the negative effect of high blood glucose levels, especially after meals, in the context of cardiovascular disease.
What all this points at is what we could call a postprandial glucose hypothesis: Lower your postprandial glucose levels, and live a longer, healthier life! And, by the way, if your postprandial glucose levels are under control, lipids do not matter much! Or maybe your lipids will fall into place, without any need for statin drugs, after your postprandial glucose levels are under control. One way or another, the outcome will be a positive one. That is what the data from this study is telling us.
How do you lower your postprandial glucose levels?
A good way to start is to remove foods rich in refined carbohydrates and sugars from your diet. Almost all of these are foods engineered by humans with the goal of being addictive; they usually come in boxes and brightly colored plastic wraps. They are not hard to miss. They are typically in the central aisles of supermarkets. The sooner you remove them from your diet, the better. The more completely you do this, the better.
Note that the evidence discussed in this post is in connection with blood glucose levels, not glucose metabolism per se. If you have impaired glucose metabolism (e.g., diabetes type 2), you can still avoid a lot of problems if you effectively control your blood glucose levels. You may have to be a bit more aggressive, adding low carbohydrate dieting (as in the Atkins or Optimal diets) to the removal of refined carbohydrates and sugars from your diet; the latter is in many ways similar to adopting a Paleolithic diet. You may have to take some drugs, such as Metformin (a.k.a. Glucophage). But you are certainly not doomed if you are diabetic.
Reference:
Leiter, L.A., Ceriello, A., Davidson, J.A., Hanefeld, M., Monnier, L., Owens, D.R., Tajima, N., & Tuomilehto, J. (2005). Postprandial glucose regulation: New data and new implications. Clinical Therapeutics, 27(2), S42-S56.
HbA1c, or glycated hemoglobin, is a measure of average blood glucose over a period of a few months. Blood glucose glycates (i.e., sticks to) hemoglobin, a protein found in red blood cells. Red blood cells are relatively long-lived, lasting approximately 3 months. Thus HbA1c (given in percentages) is a good indicator of average blood glucose levels, if you don’t suffer from anemia or a few other blood abnormalities.
Based on HbA1c, one can then estimate his or her average blood glucose level for the previous 3 months or so before the test, using one of the following equations, depending on whether the measurement is in mg/dl or mmol/l.
Average blood glucose (mg/dl) = 28.7 × HbA1c − 46.7
Average blood glucose (mmol/l) = 1.59 × HbA1c − 2.59
Elevated blood glucose levels cause damage in the body primarily through glycation, which leads to the formation of advanced glycation endproducts (AGEs). Given this, HbA1c can be seen as a proxy for the level of damage done by elevated blood glucose levels to various body tissues. This damage occurs over time; often after many years of high blood glucose levels. It includes kidney damage, neurological damage, cardiovascular damage, and damage to the retina.
Most regular blood exams focus on fasting blood glucose as a measure of glucose metabolism status. Many medical practitioners have as a target a fasting blood glucose level of 125 mg/dl (7 mmol/l) or less, and largely disregard postprandial glucose levels or HbA1c in their management of glucose metabolism. Leiter and colleagues (2005; full reference at the end of this post) showed that this focus on fasting blood glucose is a mistake. They are not alone; many others made this point, including some very knowledgeable bloggers who focus on diabetes (see “Interesting links” section of this blog). Leiter and colleagues (2005) also provided some interesting graphs and figures, including eye-opening correlations between various variables and arterial stiffness. The figure below (click to enlarge) shows the contribution of postprandial glucose to HbA1c.
Note that the lower the HbA1c is in the figure (horizontal axis), the higher is the postprandial glucose contribution to HbA1c. And, the lower the HbA1c, the closer the individuals are to what one could consider having a perfectly normal HbA1c level (around 5 percent). That is, only for individuals whose HbA1c levels are very high, fasting blood glucose levels are relatively reliable measures of the tissue damage done be elevated blood glucose levels.
The table below (click to enlarge) shows P values associated with the impact of various variables (listed on the leftmost column) on arterial stiffness. This measure, arterial stiffness, is strongly associated with an increased risk of cardiovascular events. Look at the middle column showing P values adjusted for age and height. The lower the P value, the more a variable affects arterial stiffness. The variable with the lowest P value by far is 2-hour postprandial blood glucose; the blood glucose levels measured 2 hours after meals.
Fasting glucose levels were reported to be statistically insignificant because of the P = 0.049, in terms of their effect on arterial stiffness, but this P value is actually significant, although barely, at the 0.05 level (95 percent confidence). Interestingly, the following measures are not even on the radar screen, as far as arterial stiffness is concerned: systolic blood pressure, LDL cholesterol, HDL cholesterol, triglycerides, and fasting insulin levels.
What about the lipid hypothesis, and the “bad” LDL cholesterol!? This study is telling us that these are not very relevant for arterial stiffness when we control for the effect of blood glucose measures. Not even fasting insulin levels matters much! Wait, not even HDL!!! A high HDL has been definitely shown to be protective, but when we look at the relative magnitude of various effects, the story is a bit different. A high HDL’s protective effect exists, but it is dwarfed by the negative effect of high blood glucose levels, especially after meals, in the context of cardiovascular disease.
What all this points at is what we could call a postprandial glucose hypothesis: Lower your postprandial glucose levels, and live a longer, healthier life! And, by the way, if your postprandial glucose levels are under control, lipids do not matter much! Or maybe your lipids will fall into place, without any need for statin drugs, after your postprandial glucose levels are under control. One way or another, the outcome will be a positive one. That is what the data from this study is telling us.
How do you lower your postprandial glucose levels?
A good way to start is to remove foods rich in refined carbohydrates and sugars from your diet. Almost all of these are foods engineered by humans with the goal of being addictive; they usually come in boxes and brightly colored plastic wraps. They are not hard to miss. They are typically in the central aisles of supermarkets. The sooner you remove them from your diet, the better. The more completely you do this, the better.
Note that the evidence discussed in this post is in connection with blood glucose levels, not glucose metabolism per se. If you have impaired glucose metabolism (e.g., diabetes type 2), you can still avoid a lot of problems if you effectively control your blood glucose levels. You may have to be a bit more aggressive, adding low carbohydrate dieting (as in the Atkins or Optimal diets) to the removal of refined carbohydrates and sugars from your diet; the latter is in many ways similar to adopting a Paleolithic diet. You may have to take some drugs, such as Metformin (a.k.a. Glucophage). But you are certainly not doomed if you are diabetic.
Reference:
Leiter, L.A., Ceriello, A., Davidson, J.A., Hanefeld, M., Monnier, L., Owens, D.R., Tajima, N., & Tuomilehto, J. (2005). Postprandial glucose regulation: New data and new implications. Clinical Therapeutics, 27(2), S42-S56.
Wednesday, May 26, 2010
Oven roasted meat: Pork tenderloin
This cut of pork is the equivalent in the pig of the filet mignon in cattle. It is just as soft, and lean too. A 100 g portion of roasted pork tenderloin will have about 22 g of protein, and 6 g of fat. Most of the fat will be monounsaturated and saturated, and some polyunsaturated. The latter will contain about 450 mg of omega-6 and 15 mg of omega-3 fats in it.
The saturated fat is good for you. The omega-6-to-omega-3 ratio is not a great one, but a 100 g portion will have a small absolute amount of omega-6 fats, which can be easily offset with some omega-3 from seafood or a small amount of fish oil. Pork tenderloin is easy to find in supermarkets, and is much less expensive than filet mignon, even though it is a relatively expensive cut of meat.
Below are the before and after photos of a roasted pork tenderloin we prepared and ate recently; a simple recipe follows the photos. This type of cooking leads to a Maillard reaction, which is clear from the browning of the meat and around it on the casserole. I am not too concerned; more about this after the recipe.
Below is the simple recipe. The roasted pork pieces come off easily after the roasting is done, and almost melt in the mouth!
- Make about 10 holes on 2 lbs of pork tenderloin, and add chopped garlic pieces into each of them.
- Pour about a cup of salsa evenly over the pork tenderloin pieces.
- Cover roasting container (casserole in photos) with aluminum foil to preserve moisture.
- Preheat over to 375 degrees Fahrenheit.
- Roast the pork tenderloin for about 3 hours.
Now back to the Maillard reaction. When it happens inside the body, it is a step in the formation of advanced glycation endproducts (AGEs), which is not very good, as AGEs are believed to cause accelerated aging. However, the evidence that cooked meat is unhealthy, in the absence of leaky gut problems, is very slim. There are many hypothesized causes of the leaky gut syndrome, one of which is consumption of refined wheat products.
Our Paleolithic ancestors must have eaten charred meat on a regular basis, so it does not make much evolutionary sense to think that eating roasted meat leads to accelerated aging through the ingestion of AGEs. It is possible that eating charred meat caused health problems for our ancestors, and they threw their meat in the fire and then ate it charred anyway. Perhaps the health problems caused by charring were offset by the benefits of killing parasites living in meat with the heat of fire.
This is an open issue that needs much more research. Based on most of the research I have seen so far, eating roasted meat is not even in the same universe, in terms of the health problems that it can possibly generate, as is eating foods rich in refined carbohydrates and sugars (e.g., white bread, bagels, pasta, and non-diet sodas).
The saturated fat is good for you. The omega-6-to-omega-3 ratio is not a great one, but a 100 g portion will have a small absolute amount of omega-6 fats, which can be easily offset with some omega-3 from seafood or a small amount of fish oil. Pork tenderloin is easy to find in supermarkets, and is much less expensive than filet mignon, even though it is a relatively expensive cut of meat.
Below are the before and after photos of a roasted pork tenderloin we prepared and ate recently; a simple recipe follows the photos. This type of cooking leads to a Maillard reaction, which is clear from the browning of the meat and around it on the casserole. I am not too concerned; more about this after the recipe.
Below is the simple recipe. The roasted pork pieces come off easily after the roasting is done, and almost melt in the mouth!
- Make about 10 holes on 2 lbs of pork tenderloin, and add chopped garlic pieces into each of them.
- Pour about a cup of salsa evenly over the pork tenderloin pieces.
- Cover roasting container (casserole in photos) with aluminum foil to preserve moisture.
- Preheat over to 375 degrees Fahrenheit.
- Roast the pork tenderloin for about 3 hours.
Now back to the Maillard reaction. When it happens inside the body, it is a step in the formation of advanced glycation endproducts (AGEs), which is not very good, as AGEs are believed to cause accelerated aging. However, the evidence that cooked meat is unhealthy, in the absence of leaky gut problems, is very slim. There are many hypothesized causes of the leaky gut syndrome, one of which is consumption of refined wheat products.
Our Paleolithic ancestors must have eaten charred meat on a regular basis, so it does not make much evolutionary sense to think that eating roasted meat leads to accelerated aging through the ingestion of AGEs. It is possible that eating charred meat caused health problems for our ancestors, and they threw their meat in the fire and then ate it charred anyway. Perhaps the health problems caused by charring were offset by the benefits of killing parasites living in meat with the heat of fire.
This is an open issue that needs much more research. Based on most of the research I have seen so far, eating roasted meat is not even in the same universe, in terms of the health problems that it can possibly generate, as is eating foods rich in refined carbohydrates and sugars (e.g., white bread, bagels, pasta, and non-diet sodas).
Labels:
AGEs,
pork,
recipe,
roasting,
tenderloin
Monday, May 24, 2010
Intermittent fasting, engineered foods, leptin, and ghrelin
Engineered foods are designed by smart people, and the goal is not usually to make you healthy; the goal is to sell as many units as possible. Some engineered foods are “fortified” with the goal of making them as healthy as possible. The problem is that food engineers are competing with many millions of years of evolution, and evolution usually leads to very complex metabolic processes. Evolved mechanisms tend to be redundant, leading to the interaction of many particles, enzymes, hormones etc.
Natural foods are not designed to make you eat them nonstop. Animals do not want to be eaten (even these odd-looking birds below). Most plants do not “want” their various nutritious parts to be eaten. Fruits are exceptions, but plants do not want one single individual to eat all their fruits. That compromises seed dispersion. Multiple individual fruit eaters enhance seed dispersion. Plants "want" one individual animal to eat some of their fruits and then move on, so that other individuals can also eat.
It is safe to assume that doughnut manufacturers want one single individual to eat as many doughnuts as possible, and many individuals to want to do that. That takes some serious food engineering, and a lot of testing. Success will increase the manufacturers' revenues, the real bottom line for them. The medical establishment will then take care of those individuals, and prolong their miserable lives so that they can continue eating doughnuts for as long as possible. It is self-perpetuating system.
As mentioned in this previous post, to succeed in the practice of intermittent fasting, one has to stop worrying about food, and one good step in that direction is to avoid engineered foods. In this sense, intermittent fasting can be seen as a form of liberation. Doing something enjoyable and forgetting about food. Like children playing outdoors; they do not care as much about food as they do about play. Even sleeping will do; most people forget about eating when they are asleep.
Intermittent fasting as a religious and/or social activity, as in the Great Lent and Ramadan, also seems to work well. Any activity that brings people together with a common goal, especially if the goal is not to do something evil, has a lot of potential for success.
If you approach intermittent fasting as another thing to worry about, then it will be tough – one fast per week, on the same day of the week, from 7.33 pm of one day to 3.17 pm of the next day. I exaggerate a bit. Anyway, if you approach it as another obligation, another modern stressor, you will probably fail in the medium to long term. It is just commonsense. Maybe you will be able to do it for a while, but not for long enough to reap some serious benefits. A few fasts are not going to make you lose a lot of weight; the body will adapt in a compensatory way during the fast, slowing down your metabolism a bit and conserving calories. On top of that, you will feel very, very hungry. That will make you binge when you break your fast. Compensatory adaptation (a very general phenomenon) is something that our body is very good at, regardless of what we want it to do.
From a more pragmatic perspective, for most people it is easier to fast at night and in the morning. Eating a big meal right after you wake up is not a very natural activity; several hormones that promote body fat catabolism are often elevated in the morning, causing mild physiological insulin resistance.
If you have dinner at 7 pm, skip breakfast, and then have brunch the next day at 10 am, you will have fasted for 15 h. If you skip breakfast and brunch, and have lunch at noon the next day, you will have fasted for 17 h.
On the other hand, if you have breakfast at 8 am, skip lunch, and then have dinner at 6 pm, you will have fasted only for 10 h.
Leptin levels seem to go down significantly after 12 h of fasting, leading to increased body fat catabolism and leptin sensitivity. This is a good thing, since leptin resistance seems to frequently precede insulin resistance.
Many people think that skipping breakfast will make them fat, for various reasons, including that being what sumo wrestlers do to put on enormous amounts of body fat. Well, skipping breakfast probably will make people fat if, when they break the fast, they stuff themselves to the point of almost throwing up, combine plenty of easily digestible carbohydrates (e.g., multiple bowls of rice) with a lot of dietary fat, and then go to sleep. That is what sumo wrestlers normally do.
Eating fat is great, but not together with lots of easily digestible carbohydrates. Even eating a lot of fat by itself will make it difficult for you to shed enough fat to look like the hunter-gatherers in this post. But your body fat set point will be much lower if you eat a lot of fat by itself than if you eat a lot of fat with a lot of easily digestible carbohydrates.
Anyway, if people skip breakfast and eat what they normally eat at lunch, they will not gain more body fat than they would have if they had breakfast. If they do anything to boost their metabolism in the morning, they will most certainly lose body fat in a noticeable way over several weeks, as long as they have enough fat to lose. For example, they can add some light activity in the morning (such as walking), or have a metabolism-boosting drink (e.g., coffee, green tea), or both.
Our hunter-gatherer ancestors, living outdoors, probably spent most of their day performing light activities that involved little stress. Those activities increase metabolism and fat burning, while keeping stress hormone levels at low ranges. Hunger suppression was the result, making intermittent fasting fairly easy.
Again, intermittent fasting should be approached as a form of liberation. You are no longer a slave of food.
It helps staying away from engineered foods as much as possible, because, again, they are usually engineered with food addiction in mind. I am talking primarily about foods rich in refined carbohydrates and sugars. They come in boxes and plastic bags with labels describing calories and macronutrient composition, which are often wrong or misleading.
Let us say we could transport a group of archaic Homo sapiens to a modern city, and feed them white bread, bagels, doughnuts, potato chips industrially fried in vegetable oils, and the like. Would they say “Yuck, how can these people eat this?” No, they would not. It would be heaven for them; they would want nothing else for the rest of their gustatorily happy but health-wise miserable lives.
While practicing intermittent fasting, it is probably a good idea to have fixed meal times, and skipping them from time to time. The reason is the hunger hormone ghrelin, secreted by the stomach (mostly) and pancreas to stimulate hunger and possibly prepare the digestive tract for optimal or quasi-optimal absorption of food. Its secretion appears to follow the pattern of habitual meals adopted by a person.
References:
Elliott, W.H., & Elliott, D.C. (2009). Biochemistry and molecular biology. 4th Edition. New York: NY: Oxford University Press.
Fuhrman, J., & Barnard, N.D. (1995). Fasting and eating for health: A medical doctor's program for conquering disease. New York, NY: St. Martin’s Press.
Natural foods are not designed to make you eat them nonstop. Animals do not want to be eaten (even these odd-looking birds below). Most plants do not “want” their various nutritious parts to be eaten. Fruits are exceptions, but plants do not want one single individual to eat all their fruits. That compromises seed dispersion. Multiple individual fruit eaters enhance seed dispersion. Plants "want" one individual animal to eat some of their fruits and then move on, so that other individuals can also eat.
(Source: Teamsugar.com)
It is safe to assume that doughnut manufacturers want one single individual to eat as many doughnuts as possible, and many individuals to want to do that. That takes some serious food engineering, and a lot of testing. Success will increase the manufacturers' revenues, the real bottom line for them. The medical establishment will then take care of those individuals, and prolong their miserable lives so that they can continue eating doughnuts for as long as possible. It is self-perpetuating system.
As mentioned in this previous post, to succeed in the practice of intermittent fasting, one has to stop worrying about food, and one good step in that direction is to avoid engineered foods. In this sense, intermittent fasting can be seen as a form of liberation. Doing something enjoyable and forgetting about food. Like children playing outdoors; they do not care as much about food as they do about play. Even sleeping will do; most people forget about eating when they are asleep.
Intermittent fasting as a religious and/or social activity, as in the Great Lent and Ramadan, also seems to work well. Any activity that brings people together with a common goal, especially if the goal is not to do something evil, has a lot of potential for success.
If you approach intermittent fasting as another thing to worry about, then it will be tough – one fast per week, on the same day of the week, from 7.33 pm of one day to 3.17 pm of the next day. I exaggerate a bit. Anyway, if you approach it as another obligation, another modern stressor, you will probably fail in the medium to long term. It is just commonsense. Maybe you will be able to do it for a while, but not for long enough to reap some serious benefits. A few fasts are not going to make you lose a lot of weight; the body will adapt in a compensatory way during the fast, slowing down your metabolism a bit and conserving calories. On top of that, you will feel very, very hungry. That will make you binge when you break your fast. Compensatory adaptation (a very general phenomenon) is something that our body is very good at, regardless of what we want it to do.
From a more pragmatic perspective, for most people it is easier to fast at night and in the morning. Eating a big meal right after you wake up is not a very natural activity; several hormones that promote body fat catabolism are often elevated in the morning, causing mild physiological insulin resistance.
If you have dinner at 7 pm, skip breakfast, and then have brunch the next day at 10 am, you will have fasted for 15 h. If you skip breakfast and brunch, and have lunch at noon the next day, you will have fasted for 17 h.
On the other hand, if you have breakfast at 8 am, skip lunch, and then have dinner at 6 pm, you will have fasted only for 10 h.
Leptin levels seem to go down significantly after 12 h of fasting, leading to increased body fat catabolism and leptin sensitivity. This is a good thing, since leptin resistance seems to frequently precede insulin resistance.
Many people think that skipping breakfast will make them fat, for various reasons, including that being what sumo wrestlers do to put on enormous amounts of body fat. Well, skipping breakfast probably will make people fat if, when they break the fast, they stuff themselves to the point of almost throwing up, combine plenty of easily digestible carbohydrates (e.g., multiple bowls of rice) with a lot of dietary fat, and then go to sleep. That is what sumo wrestlers normally do.
Eating fat is great, but not together with lots of easily digestible carbohydrates. Even eating a lot of fat by itself will make it difficult for you to shed enough fat to look like the hunter-gatherers in this post. But your body fat set point will be much lower if you eat a lot of fat by itself than if you eat a lot of fat with a lot of easily digestible carbohydrates.
Anyway, if people skip breakfast and eat what they normally eat at lunch, they will not gain more body fat than they would have if they had breakfast. If they do anything to boost their metabolism in the morning, they will most certainly lose body fat in a noticeable way over several weeks, as long as they have enough fat to lose. For example, they can add some light activity in the morning (such as walking), or have a metabolism-boosting drink (e.g., coffee, green tea), or both.
Our hunter-gatherer ancestors, living outdoors, probably spent most of their day performing light activities that involved little stress. Those activities increase metabolism and fat burning, while keeping stress hormone levels at low ranges. Hunger suppression was the result, making intermittent fasting fairly easy.
Again, intermittent fasting should be approached as a form of liberation. You are no longer a slave of food.
It helps staying away from engineered foods as much as possible, because, again, they are usually engineered with food addiction in mind. I am talking primarily about foods rich in refined carbohydrates and sugars. They come in boxes and plastic bags with labels describing calories and macronutrient composition, which are often wrong or misleading.
Let us say we could transport a group of archaic Homo sapiens to a modern city, and feed them white bread, bagels, doughnuts, potato chips industrially fried in vegetable oils, and the like. Would they say “Yuck, how can these people eat this?” No, they would not. It would be heaven for them; they would want nothing else for the rest of their gustatorily happy but health-wise miserable lives.
While practicing intermittent fasting, it is probably a good idea to have fixed meal times, and skipping them from time to time. The reason is the hunger hormone ghrelin, secreted by the stomach (mostly) and pancreas to stimulate hunger and possibly prepare the digestive tract for optimal or quasi-optimal absorption of food. Its secretion appears to follow the pattern of habitual meals adopted by a person.
References:
Elliott, W.H., & Elliott, D.C. (2009). Biochemistry and molecular biology. 4th Edition. New York: NY: Oxford University Press.
Fuhrman, J., & Barnard, N.D. (1995). Fasting and eating for health: A medical doctor's program for conquering disease. New York, NY: St. Martin’s Press.
Sunday, May 23, 2010
Gallstone Development
Gallstones develop in the gallbladder, a small organ that stores and releases the bile made by the liver. Bile is a dark green fluid containing bile salts and cholesterol. The gallbladder releases bile into the small intestine to assist in digesting fats more efficiently. However, if the bile is contains high concentrations of cholesterol, then stones too difficult for the bile salts to dissolve may develop (1).
Losing weight too quickly or fasting can cause development of gallstones. The quick weight loss and fasting is thought to disturb the balance of bile salts and cholesterol (2;3).
The risk may increase if consuming a diet too low in fat. Avoiding fat reduces frequency of gallbladder emptying. This, in turn, may cause cholesterol to accumulate and lead to greater risk of forming stones (3;4).
References
1. Dowling RH. Review: pathogenesis of gallstones. Aliment Pharmacol Ther 2000;14 Suppl 2:39-47.
2. Wudel LJ, Jr., Wright JK, Debelak JP, Allos TM, Shyr Y, Chapman WC. Prevention of gallstone formation in morbidly obese patients undergoing rapid weight loss: results of a randomized controlled pilot study. J Surg Res 2002;102:50-6.
3. Festi D, Colecchia A, Orsini M et al. Gallbladder motility and gallstone formation in obese patients following very low calorie diets. Use it (fat) to lose it (well). Int J Obes Relat Metab Disord 1998;22:592-600.
4. Vezina WC, Grace DM, Hutton LC et al. Similarity in gallstone formation from 900 kcal/day diets containing 16 g vs 30 g of daily fat: evidence that fat restriction is not the main culprit of cholelithiasis during rapid weight reduction. Dig Dis Sci 1998;43:554-61.
Losing weight too quickly or fasting can cause development of gallstones. The quick weight loss and fasting is thought to disturb the balance of bile salts and cholesterol (2;3).
The risk may increase if consuming a diet too low in fat. Avoiding fat reduces frequency of gallbladder emptying. This, in turn, may cause cholesterol to accumulate and lead to greater risk of forming stones (3;4).
References
1. Dowling RH. Review: pathogenesis of gallstones. Aliment Pharmacol Ther 2000;14 Suppl 2:39-47.
2. Wudel LJ, Jr., Wright JK, Debelak JP, Allos TM, Shyr Y, Chapman WC. Prevention of gallstone formation in morbidly obese patients undergoing rapid weight loss: results of a randomized controlled pilot study. J Surg Res 2002;102:50-6.
3. Festi D, Colecchia A, Orsini M et al. Gallbladder motility and gallstone formation in obese patients following very low calorie diets. Use it (fat) to lose it (well). Int J Obes Relat Metab Disord 1998;22:592-600.
4. Vezina WC, Grace DM, Hutton LC et al. Similarity in gallstone formation from 900 kcal/day diets containing 16 g vs 30 g of daily fat: evidence that fat restriction is not the main culprit of cholelithiasis during rapid weight reduction. Dig Dis Sci 1998;43:554-61.
Labels:
nutr therap
Homocysteinemia and Pernicious anemia
Pernicious anemia, a megaloblastic anemia caused by B12 deficiency, is associated with hyperhomocysteinemia. Because B12 is needed for methionine synthase to methylate homocysteine to methionine, a deficiency causes an accumulation of both homocysteine and methylmalonic acid (1). When both are elevated, marking the pernicious anemia, it can lead to progressive demyelination and neurological deterioration.
A folate deficiency may also result in megaloblastic anemia. If homocysteine is elevated but not methylmalonic acid, then the result is probably a folate deficiency. It is important for treatment to be correct. Large doses of folate can correct, or "mask," symptoms of pernicious anemia, which can result in irreversible neuropathy (2).
References
1. Devlin TM. Textbook of Biochemistry with Clinical Correlations. Philadelphia: Wiley-Liss, 2002
2. Pagana, K.D., Pagana, T.J. Mostby's Manual of Diagnostic and Laboratory Tests, 3rd ed. Mosby Elsvier, 2006
A folate deficiency may also result in megaloblastic anemia. If homocysteine is elevated but not methylmalonic acid, then the result is probably a folate deficiency. It is important for treatment to be correct. Large doses of folate can correct, or "mask," symptoms of pernicious anemia, which can result in irreversible neuropathy (2).
References
1. Devlin TM. Textbook of Biochemistry with Clinical Correlations. Philadelphia: Wiley-Liss, 2002
2. Pagana, K.D., Pagana, T.J. Mostby's Manual of Diagnostic and Laboratory Tests, 3rd ed. Mosby Elsvier, 2006
Labels:
nutr therap
Saturday, May 22, 2010
Before Taking a Statin, Read This
I thought this was an interesting article from Businessweek a couple of years ago and was blown away by the numbers showing that few people actually receive any benefit from statins.
If you don't read it, then here are a few tidbits from the article that I thought would give it to you in a nutshell:
If you don't read it, then here are a few tidbits from the article that I thought would give it to you in a nutshell:
- ...for every 100 people in the trial, which lasted 3 1/3 years, three people on placebos and two people on Lipitor had heart attacks. The difference credited to the drug? One fewer heart attack per 100 people. So to spare one person a heart attack, 100 people had to take Lipitor for more than three years. The other 99 got no measurable benefit.
- ...an estimated 10% to 15% of statin users suffer side effects, including muscle pain, cognitive impairments, and sexual dysfunction
- "There's a tendency to assume drugs work really well, but people would be surprised by the actual magnitude of the benefits,"
- For anyone worried about heart disease, the first step should always be a better diet and increased physical activity. Do that, and "we would cut the number of people at risk so dramatically" that far fewer drugs would be needed...
- "The way our health-care system runs, it is not based on data, it is based on what makes money."
Friday, May 21, 2010
Predicting a Heart Attack with CRP
Currently, the existing biomarkers for a cardiac event include B-type natriuretic peptide, tro-ponins and C-reactive protein. C-reactive protein is an acute-phase protein released in response to inflammation.
Recently, the development of a high-sensitivity assay for CRP (hs-CRP) has been made available. The assay works because it can accurately reflect even low levels of CRP. There have been quite a few prospective studies that have shown that an assay of a baseline CRP can be used as a marker for cardiovascular events.
When patients have a test that shows elevated levels, it is even a better marker than LDL cholesterol for predicting events such as myocardial infarction. An elevated test, however, can also mean hypertension, metabolic syndrome or diabetes, or a chronic infection.
In addition, Lipoprotein (a), or Lp(a), when combined with C-reactive protein, can increase the predictive value of a cardiac event. This is especially true for those who have normal cholesterol levels. The reason is that the lipoprotein promotes vascular inflammation that affects the atherogenic process directly.
Reference
Pagana, K.D., Pagana, T.J. Mosby's Manual of Diagnostic and Laboratory Tests, 3rd ed. Mosby Elsvier, 2006.
Recently, the development of a high-sensitivity assay for CRP (hs-CRP) has been made available. The assay works because it can accurately reflect even low levels of CRP. There have been quite a few prospective studies that have shown that an assay of a baseline CRP can be used as a marker for cardiovascular events.
When patients have a test that shows elevated levels, it is even a better marker than LDL cholesterol for predicting events such as myocardial infarction. An elevated test, however, can also mean hypertension, metabolic syndrome or diabetes, or a chronic infection.
In addition, Lipoprotein (a), or Lp(a), when combined with C-reactive protein, can increase the predictive value of a cardiac event. This is especially true for those who have normal cholesterol levels. The reason is that the lipoprotein promotes vascular inflammation that affects the atherogenic process directly.
Reference
Pagana, K.D., Pagana, T.J. Mosby's Manual of Diagnostic and Laboratory Tests, 3rd ed. Mosby Elsvier, 2006.
Labels:
nutr therap
How to Rid Yourself of Statin-induced Muscle Pain
When a patient is on a statin, nutritionists should advise that they don’t have to suffer from the side effects of statin-associated muscle pain (myalgia). Studies are showing that supplementation with two key compounds are useful for decreasing the pain. The first is ubiquinone (coenzyme Q10, coQ10) and the other is cholecalciferol (vitamin D3).
Statins such as Lipitor, Zocor and Mevacor reduce cholesterol synthesis by directly inhibiting the enzyme HMG-CoA reductase and deplete production of its product, mevalonate (1). Mevalonite, however, is also the precursor to coQ10 and squalene. Both of these are vital nutrients with profound effects on the body.
CoQ10
CoQ10 is a lipid-soluble antioxidant playing a protective effect in the membranes of every cell in the body. In that capacity, it serves to protect against oxidative damage to cells. Equally important, the compound is necessary for electron transfer in the mitochondrial electron transport chain for producing energy (2). Without it, our muscles could not function in their full capacity.
Supplementation with coQ10 combined with statin treatment helps reduce muscle pain (not to mention improve energy levels). According to a double-blind study in 2007 at Stony Brook University, which compared coQ10 supplementation (100mg/d) with vitamin E (400 IU/d), showed that patients taking the coQ10 had 40 percent decrease in the severity of their pain (3).
Vitamin D
Squalene is important because it is the precursor for 25 hydroxyvitamin D (25(OH)D) as well as other steroid hormones. For this reason that, it is suggested that statin drugs can lead to 25(OH)D insufficiency or deficiency. Vitamin D is not only critical for speeding up calcium absorption for bone health, but emerging studies are finding that it’s also vital for the health of muscles (4).
Low vitamin D levels are also associated with statin-induced muscle pain. When researchers from the Cholesterol Center at the Jewish Hospital in Cincinnatti in Ohio treated myalgia in 38 statin-treated patients with vitamin D (50,000 IU/week for 12 weeks), 35 of the patients experienced 92 percent reduction in pain symptoms (5).
Reducing muscle pain with supplementation
If you must take a statin, then supplementation can be to your advantage. As in the studies, supplementation with coQ10 at 100 mg in an absorbable form can potentially help to keep pain under control by replenishing coQ10 that is lost. In addition, keeping 25(OH)D to levels in the plasma to “sufficient” amounts (32 ng/mL) through supplementation with vitamin D and sensible sun exposure can go far to reduce pain.
Reference List
1. Scharnagl H, Marz W. New lipid-lowering agents acting on LDL receptors. Curr Top Med Chem 2005;5:233-42.
2. Jeya M, Moon HJ, Lee JL, Kim IW, Lee JK. Current state of coenzyme Q(10) production and its applications. Appl Microbiol Biotechnol 2010;85:1653-63.
3. Caso G, Kelly P, McNurlan MA, Lawson WE. Effect of coenzyme q10 on myopathic symptoms in patients treated with statins. Am J Cardiol 2007;99:1409-12.
4. Visvanathan R, Chapman I. Preventing sarcopaenia in older people. Maturitas 2010.
5. Ahmed W, Khan N, Glueck CJ et al. Low serum 25 (OH) vitamin D levels (<32 ng/mL) are associated with reversible myositis-myalgia in statin-treated patients. Transl Res 2009;153:11-6.
Statins such as Lipitor, Zocor and Mevacor reduce cholesterol synthesis by directly inhibiting the enzyme HMG-CoA reductase and deplete production of its product, mevalonate (1). Mevalonite, however, is also the precursor to coQ10 and squalene. Both of these are vital nutrients with profound effects on the body.
CoQ10
CoQ10 is a lipid-soluble antioxidant playing a protective effect in the membranes of every cell in the body. In that capacity, it serves to protect against oxidative damage to cells. Equally important, the compound is necessary for electron transfer in the mitochondrial electron transport chain for producing energy (2). Without it, our muscles could not function in their full capacity.
Supplementation with coQ10 combined with statin treatment helps reduce muscle pain (not to mention improve energy levels). According to a double-blind study in 2007 at Stony Brook University, which compared coQ10 supplementation (100mg/d) with vitamin E (400 IU/d), showed that patients taking the coQ10 had 40 percent decrease in the severity of their pain (3).
Vitamin D
Squalene is important because it is the precursor for 25 hydroxyvitamin D (25(OH)D) as well as other steroid hormones. For this reason that, it is suggested that statin drugs can lead to 25(OH)D insufficiency or deficiency. Vitamin D is not only critical for speeding up calcium absorption for bone health, but emerging studies are finding that it’s also vital for the health of muscles (4).
Low vitamin D levels are also associated with statin-induced muscle pain. When researchers from the Cholesterol Center at the Jewish Hospital in Cincinnatti in Ohio treated myalgia in 38 statin-treated patients with vitamin D (50,000 IU/week for 12 weeks), 35 of the patients experienced 92 percent reduction in pain symptoms (5).
Reducing muscle pain with supplementation
If you must take a statin, then supplementation can be to your advantage. As in the studies, supplementation with coQ10 at 100 mg in an absorbable form can potentially help to keep pain under control by replenishing coQ10 that is lost. In addition, keeping 25(OH)D to levels in the plasma to “sufficient” amounts (32 ng/mL) through supplementation with vitamin D and sensible sun exposure can go far to reduce pain.
Reference List
1. Scharnagl H, Marz W. New lipid-lowering agents acting on LDL receptors. Curr Top Med Chem 2005;5:233-42.
2. Jeya M, Moon HJ, Lee JL, Kim IW, Lee JK. Current state of coenzyme Q(10) production and its applications. Appl Microbiol Biotechnol 2010;85:1653-63.
3. Caso G, Kelly P, McNurlan MA, Lawson WE. Effect of coenzyme q10 on myopathic symptoms in patients treated with statins. Am J Cardiol 2007;99:1409-12.
4. Visvanathan R, Chapman I. Preventing sarcopaenia in older people. Maturitas 2010.
5. Ahmed W, Khan N, Glueck CJ et al. Low serum 25 (OH) vitamin D levels (<32 ng/mL) are associated with reversible myositis-myalgia in statin-treated patients. Transl Res 2009;153:11-6.
Labels:
nutr therap
Atheism is a recent Neolithic invention: Ancestral humans were spiritual people
For the sake of simplicity, this post treats “atheism” as synonymous with “non-spiritualism”. Technically, one can be spiritual and not believe in any deity or supernatural being, although this is not very common. This post argues that atheism is a recent Neolithic invention; an invention that is poorly aligned with our Paleolithic ancestry.
Our Paleolithic ancestors were likely very spiritual people; at least those belonging to the Homo sapiens species. Earlier ancestors, such as the Australopithecines, may have lacked enough intelligence to be spiritual. Interestingly, often atheism is associated with high intelligence and a deep understanding of science. Many well-known, and brilliant, evolution researchers are atheists (e.g., Richard Dawkins).
Well, when we look at our ancestors, spirituality seems to have emerged as a result of increased intelligence.
Spirituality can be seen in cave paintings, such as the one below, from the Chauvet Cave in southern France. The Chauvet Cave is believed to have the earliest known cave paintings, dating back to about 30 to 40 thousand years ago. The painting below is on the cover of the book Dawn of art: The Chauvet Cave. (See the full reference for this publication and others at the end of this post.)
The most widely accepted theory of the origin of cave paintings is that they were used in shamanic or religious rituals. By and large, they were not used to convey information (e.g., as maps); and they are often found deep in caves, in areas that are almost inaccessible, ruling out a “decorative” artistic purpose. As De La Croix and colleagues (1991) note:
Isolated hunter-gatherers also provide a glimpse at our spiritual Paleolithic past. No isolated hunter-gatherer group has ever been found in which atheism was the predominant belief among its members. In fact, the life of most isolated hunter-gatherer groups that have been studied appears to have revolved around religious rituals. In many of these groups, shamans held a very high social status, and strongly influenced group decisions.
Finally, there is solid empirical evidence from human genetics and the study of modern human groups that: (a) “religiosity” may be coded into our genes, to a larger extent in some individuals than in others; and (b) those who are spiritual, particularly those who belong to a spiritual or religious group, have generally better health and experience lower levels of depression and stress (which likely influence health) than those who do not.
There was once an ape that became smart. It invented weapons, which greatly multiplied the potential for death and destruction of the ape’s natural propensity toward violence; violence often motivated by different religious and cultural beliefs held by different groups. It also invented delicious foods rich in refined carbohydrates and sugars, which slowly poisoned the ape’s body.
Could the recent invention of atheism have been just as unhealthy?
Surely religion has been at the source of conflicts that have caused much death and destruction. But is religion, or spirituality, really to be blamed? Many other factors can lead to a great deal of death and destruction, sometimes directly, other times indirectly – e.g., poverty and illiteracy.
References:
Brown, D.E. (1991). Human universals. New York, NY: McGraw-Hill.
Chauvet, J.M., Deschamps, E.B., & Hillaire, C. (1996). Dawn of art: The Chauvet Cave. New York, NY: Harry N. Abrams.
De La Croix, H., Tansey, R.G., & Kirkpatrick, D. (1991). Gardner’s art through the ages: Ancient, medieval, and non-European art. Philadelphia, PA: Harcourt Brace.
Gombrich, E.H. (2006). The story of art. London, England: Pheidon Press.
Murdock, G.P. (1958). Outline of world cultures. New Haven, CN: Human Relations Area Files Press.
Our Paleolithic ancestors were likely very spiritual people; at least those belonging to the Homo sapiens species. Earlier ancestors, such as the Australopithecines, may have lacked enough intelligence to be spiritual. Interestingly, often atheism is associated with high intelligence and a deep understanding of science. Many well-known, and brilliant, evolution researchers are atheists (e.g., Richard Dawkins).
Well, when we look at our ancestors, spirituality seems to have emerged as a result of increased intelligence.
Spirituality can be seen in cave paintings, such as the one below, from the Chauvet Cave in southern France. The Chauvet Cave is believed to have the earliest known cave paintings, dating back to about 30 to 40 thousand years ago. The painting below is on the cover of the book Dawn of art: The Chauvet Cave. (See the full reference for this publication and others at the end of this post.)
The most widely accepted theory of the origin of cave paintings is that they were used in shamanic or religious rituals. By and large, they were not used to convey information (e.g., as maps); and they are often found deep in caves, in areas that are almost inaccessible, ruling out a “decorative” artistic purpose. As De La Croix and colleagues (1991) note:
Researchers have evidence that the hunters in the caves, perhaps in a frenzy stimulated by magical rites and dances, treated the painted animals as if they were alive. Not only was the quarry often painted as pierced by arrows, but hunters actually may have thrown spears at the images, as sharp gouges in the side of the bison at Niaux suggest.Niaux is another cave in southern France. Like the Chauvet Cave, it is full of prehistoric paintings. Even though those paintings are believed to be more recent, dating back to the end of the Paleolithic, they follow the same patterns seen almost everywhere in prehistoric art. The patterns point at a life that gravitates around spiritual rituals.
Isolated hunter-gatherers also provide a glimpse at our spiritual Paleolithic past. No isolated hunter-gatherer group has ever been found in which atheism was the predominant belief among its members. In fact, the life of most isolated hunter-gatherer groups that have been studied appears to have revolved around religious rituals. In many of these groups, shamans held a very high social status, and strongly influenced group decisions.
Finally, there is solid empirical evidence from human genetics and the study of modern human groups that: (a) “religiosity” may be coded into our genes, to a larger extent in some individuals than in others; and (b) those who are spiritual, particularly those who belong to a spiritual or religious group, have generally better health and experience lower levels of depression and stress (which likely influence health) than those who do not.
There was once an ape that became smart. It invented weapons, which greatly multiplied the potential for death and destruction of the ape’s natural propensity toward violence; violence often motivated by different religious and cultural beliefs held by different groups. It also invented delicious foods rich in refined carbohydrates and sugars, which slowly poisoned the ape’s body.
Could the recent invention of atheism have been just as unhealthy?
Surely religion has been at the source of conflicts that have caused much death and destruction. But is religion, or spirituality, really to be blamed? Many other factors can lead to a great deal of death and destruction, sometimes directly, other times indirectly – e.g., poverty and illiteracy.
References:
Brown, D.E. (1991). Human universals. New York, NY: McGraw-Hill.
Chauvet, J.M., Deschamps, E.B., & Hillaire, C. (1996). Dawn of art: The Chauvet Cave. New York, NY: Harry N. Abrams.
De La Croix, H., Tansey, R.G., & Kirkpatrick, D. (1991). Gardner’s art through the ages: Ancient, medieval, and non-European art. Philadelphia, PA: Harcourt Brace.
Gombrich, E.H. (2006). The story of art. London, England: Pheidon Press.
Murdock, G.P. (1958). Outline of world cultures. New Haven, CN: Human Relations Area Files Press.
Thursday, May 20, 2010
Cheese’s vitamin K2 content, pasteurization, and beneficial enzymes: Comments by Jack C.
The text below is all from commenter Jack C.’s notes on this post summarizing research on cheese. My additions are within “[ ]”. While the comments are there under the previous post for everyone to see, I thought that they should be in a separate post. Among other things, they provide an explanation for the findings summarized in the previous post.
During [the] cheese fermentation process the vitamin K2 (menaquinone) content of cheese is increased more than ten-fold. Vitamin K2 is anti-carcinogenic, reduces calcification of soft tissue (like arteries) and reduces bone fracture risk. So vitamin K2 in aged cheese provides major health benefits that are not present in the control nutrients. [Jack is referring to the control nutrients used in the study summarized in the previous post.]
Another apparent benefit of aged cheese is the breakdown of the peptide BCM7 (beta-casomorphin 7) which is present in the casein milk of most cows (a1 milk) in the U.S. BCM7 is a powerful oxidant and is highly atherogenic. (From "Devil in the Milk" by Keith Woodford.)
[P]asteurization is not necessary, for during the aging process, the production of lactic acid results in a drop in pH which destroys pathogenic bacteria but does not harm beneficial bacteria! Many benefits result.
In making aged cheese, the temperature [should] be kept to no more than 102 degrees F, the same temperature that the milk comes out of the cow. The many beneficial enzymes in milk (8 actually) therefore are not harmed and provide many health benefits. Lactoferrin, for example, destroys pathogenic bacteria by binding to iron (most pathogenic bacteria are iron loving) and also helps in absorption of iron. Lipase helps break down fats and reduces the load on the pancreas which produces lipase.
By federal law, milk that has not been pasteurized cannot be shipped across state lines [in the U.S.], but raw milk cheese can be legally shipped provided that it has been aged at least sixty days. Thus, in backward states like Alabama where I live that do not permit the sale of raw milk, you can get the same beneficial enzymes (well, almost) from aged cheese as from raw milk. And as you pointed out, cheese that is shrink-wrapped will keep a long time and can be easily shipped.
I buy most of my raw milk cheese from a small dairy in Elberta, Alabama, Sweet Home Farm, which produces a great variety of organic raw milk cheese from Guernsey cows that are fed nothing but grass. No grain, no antibiotics or growth hormones. There is nothing comparable in the way of milk that is available legally. The so called “organic” milk sold in stores is all ultra-pasteurized. Yuck.
Raw milk cheese is readily shipped. Sweet Home Farm does not ship cheese, so I have to go get it, 70 miles round trip. On occasion I buy raw milk cheese from Next Generation Dairy, a small coop in Minn. which promises that they do not raise the temperature of the cheese to more than 102 degrees F during manufacture. The cheese is modestly priced and can be shipped inexpensively.
Jack
***
During [the] cheese fermentation process the vitamin K2 (menaquinone) content of cheese is increased more than ten-fold. Vitamin K2 is anti-carcinogenic, reduces calcification of soft tissue (like arteries) and reduces bone fracture risk. So vitamin K2 in aged cheese provides major health benefits that are not present in the control nutrients. [Jack is referring to the control nutrients used in the study summarized in the previous post.]
Another apparent benefit of aged cheese is the breakdown of the peptide BCM7 (beta-casomorphin 7) which is present in the casein milk of most cows (a1 milk) in the U.S. BCM7 is a powerful oxidant and is highly atherogenic. (From "Devil in the Milk" by Keith Woodford.)
[P]asteurization is not necessary, for during the aging process, the production of lactic acid results in a drop in pH which destroys pathogenic bacteria but does not harm beneficial bacteria! Many benefits result.
In making aged cheese, the temperature [should] be kept to no more than 102 degrees F, the same temperature that the milk comes out of the cow. The many beneficial enzymes in milk (8 actually) therefore are not harmed and provide many health benefits. Lactoferrin, for example, destroys pathogenic bacteria by binding to iron (most pathogenic bacteria are iron loving) and also helps in absorption of iron. Lipase helps break down fats and reduces the load on the pancreas which produces lipase.
By federal law, milk that has not been pasteurized cannot be shipped across state lines [in the U.S.], but raw milk cheese can be legally shipped provided that it has been aged at least sixty days. Thus, in backward states like Alabama where I live that do not permit the sale of raw milk, you can get the same beneficial enzymes (well, almost) from aged cheese as from raw milk. And as you pointed out, cheese that is shrink-wrapped will keep a long time and can be easily shipped.
I buy most of my raw milk cheese from a small dairy in Elberta, Alabama, Sweet Home Farm, which produces a great variety of organic raw milk cheese from Guernsey cows that are fed nothing but grass. No grain, no antibiotics or growth hormones. There is nothing comparable in the way of milk that is available legally. The so called “organic” milk sold in stores is all ultra-pasteurized. Yuck.
Raw milk cheese is readily shipped. Sweet Home Farm does not ship cheese, so I have to go get it, 70 miles round trip. On occasion I buy raw milk cheese from Next Generation Dairy, a small coop in Minn. which promises that they do not raise the temperature of the cheese to more than 102 degrees F during manufacture. The cheese is modestly priced and can be shipped inexpensively.
Jack
Tuesday, May 18, 2010
Cheese consumption, visceral fat, and adiponectin levels
Several bacteria feed on lactose, the sugar found in milk, producing cheese for us as a byproduct of their feeding. This is why traditionally made cheese can be eaten by those who are lactose intolerant. Cheese consumption predates written history. This of course does not refer to processed cheese, frequently sold under the name “American cheese”. Technically speaking, processed cheese is not “real” cheese.
One reasonably reliable way of differentiating between traditional and processed cheese varieties is to look for holes. Cheese-making bacteria produce a gas, carbon dioxide, which leaves holes in cheese. There are exceptions though, and sometimes the holes are very small, giving the impression of no holes. Another good way is to look at the label and the price; usually processed cheese is labeled as such, and is cheaper than traditionally made cheese.
Cheese does not normally spoil; it ages. When vacuum-wrapped, cheese is essentially in “suspended animation”. After opening it, it is a good idea to store it in such a way as to allow it to “breathe”, or continue aging. Wax paper does a fine job at that. This property, extended aging, has made cheese a very useful source of nutrition for travelers in ancient times. It was reportedly consumed in large quantities by Roman soldiers.
Walther and colleagues (2008) provide a good review of the role of cheese in nutrition and health. The full reference is at the end of this post. They point out empirical evidence that cheese, particularly that produced with Lactobacillus helveticus (e.g., Gouda and Swiss cheese), contributes to lowering blood pressure, stimulates growth and development of lean body tissues (e.g., muscle), and has anti-carcinogenic properties.
The health-promoting effects of cheese were also reviewed by Higurashi and colleagues (2007), who hypothesized that those effects may be in part due to the intermediate positive effects of cheese on adiponectin and visceral body fat levels. They conducted a study with rats that supports those hypotheses.
In the study, they fed two groups of rats an isocaloric diet with 20 percent of fat, 20 percent of protein, and 60 percent of carbohydrate (in the form of sucrose). In one group, the treatment group, Gouda cheese (produced with Lactobacillus helveticus) was the main source of protein. In the other group, the control group, isolated casein was the main source of protein. The researchers were careful to avoid confounding variables; e.g., they adjusted the vitamin and mineral intake in the groups so as to match them.
The table below (click to enlarge) shows initial and final body weight, liver weight, and abdominal fat for both groups of rats. As you can see, the rats more than quadrupled in weight by the end of the 8-weight experiment! Abdominal fat was lower in the cheese group; one type of visceral fat, mesenteric, was significantly lower. Whole body weight-adjusted liver weight was higher in the cheese group. Liver weight increase is often associated with increased muscle mass. The rats in the cheese group were a little heavier on average, even though they had less abdominal fat.
The figure below shows adiponectin levels at the 4-week and 8-week marks. While adiponectin levels decreased in both groups, which was to be expected given the massive gain in weight (and probably body fat mass), only in the casein group the decrease in adiponectin was significant. In fact, the relatively small decrease in the cheese group is a bit surprising given the increase in weight observed.
If we could extrapolate these findings to humans, and this is a big “if”, one could argue that cheese has some significant health-promoting effects. There is one small problem with this study though. To ensure that the rats consumed the same number of calories, the rats in the casein group were fed slightly more sucrose. The difference was very small though; arguably not enough to explain the final outcomes.
This study is interesting because the main protein in cheese is actually casein, and also because casein powders are often favored by those wanting to put on muscle as part of a weight training program. This study suggests that the cheese-ripening process induced by Lactobacillus helveticus may yield compounds that are particularly health-promoting in three main ways – maintaining adiponectin levels; possibly increasing muscle mass; and reducing visceral fat gain, even in the presence of significant weight gain. In humans, reduced circulating adiponectin and increased visceral fat are strongly associated with the metabolic syndrome.
One caveat: if you think that eating cheese may help wipe out that stubborn abdominal fat, think again. This is a topic for another post. But, briefly, this study suggests that cheese consumption may help reduce visceral fat. Visceral fat, however, is generally fairly easy to mobilize (i.e., burn); much easier than the stubborn subcutaneous body fat that accumulates in the lower abdomen of middle-aged men and women. In middle-aged women, stubborn subcutaneous fat also accumulates in the hips and thighs.
Could eating Gouda cheese, together with other interventions (e.g., exercise), become a new weapon against the metabolic syndrome?
References:
Higurashi, S., Kunieda, Y., Matsuyama, H., & Kawakami, H. (2007). Effect of cheese consumption on the accumulation of abdominal adipose and decrease in serum adiponectin levels in rats fed a calorie dense diet. International Dairy Journal, 17(10), 1224–1231.
Walther, B., Schmid, A., Sieber, R., & Wehrmüller, K. (2008). Cheese in nutrition and health. Dairy Science Technology, 88(4), 389-405.
One reasonably reliable way of differentiating between traditional and processed cheese varieties is to look for holes. Cheese-making bacteria produce a gas, carbon dioxide, which leaves holes in cheese. There are exceptions though, and sometimes the holes are very small, giving the impression of no holes. Another good way is to look at the label and the price; usually processed cheese is labeled as such, and is cheaper than traditionally made cheese.
Cheese does not normally spoil; it ages. When vacuum-wrapped, cheese is essentially in “suspended animation”. After opening it, it is a good idea to store it in such a way as to allow it to “breathe”, or continue aging. Wax paper does a fine job at that. This property, extended aging, has made cheese a very useful source of nutrition for travelers in ancient times. It was reportedly consumed in large quantities by Roman soldiers.
Walther and colleagues (2008) provide a good review of the role of cheese in nutrition and health. The full reference is at the end of this post. They point out empirical evidence that cheese, particularly that produced with Lactobacillus helveticus (e.g., Gouda and Swiss cheese), contributes to lowering blood pressure, stimulates growth and development of lean body tissues (e.g., muscle), and has anti-carcinogenic properties.
The health-promoting effects of cheese were also reviewed by Higurashi and colleagues (2007), who hypothesized that those effects may be in part due to the intermediate positive effects of cheese on adiponectin and visceral body fat levels. They conducted a study with rats that supports those hypotheses.
In the study, they fed two groups of rats an isocaloric diet with 20 percent of fat, 20 percent of protein, and 60 percent of carbohydrate (in the form of sucrose). In one group, the treatment group, Gouda cheese (produced with Lactobacillus helveticus) was the main source of protein. In the other group, the control group, isolated casein was the main source of protein. The researchers were careful to avoid confounding variables; e.g., they adjusted the vitamin and mineral intake in the groups so as to match them.
The table below (click to enlarge) shows initial and final body weight, liver weight, and abdominal fat for both groups of rats. As you can see, the rats more than quadrupled in weight by the end of the 8-weight experiment! Abdominal fat was lower in the cheese group; one type of visceral fat, mesenteric, was significantly lower. Whole body weight-adjusted liver weight was higher in the cheese group. Liver weight increase is often associated with increased muscle mass. The rats in the cheese group were a little heavier on average, even though they had less abdominal fat.
The figure below shows adiponectin levels at the 4-week and 8-week marks. While adiponectin levels decreased in both groups, which was to be expected given the massive gain in weight (and probably body fat mass), only in the casein group the decrease in adiponectin was significant. In fact, the relatively small decrease in the cheese group is a bit surprising given the increase in weight observed.
If we could extrapolate these findings to humans, and this is a big “if”, one could argue that cheese has some significant health-promoting effects. There is one small problem with this study though. To ensure that the rats consumed the same number of calories, the rats in the casein group were fed slightly more sucrose. The difference was very small though; arguably not enough to explain the final outcomes.
This study is interesting because the main protein in cheese is actually casein, and also because casein powders are often favored by those wanting to put on muscle as part of a weight training program. This study suggests that the cheese-ripening process induced by Lactobacillus helveticus may yield compounds that are particularly health-promoting in three main ways – maintaining adiponectin levels; possibly increasing muscle mass; and reducing visceral fat gain, even in the presence of significant weight gain. In humans, reduced circulating adiponectin and increased visceral fat are strongly associated with the metabolic syndrome.
One caveat: if you think that eating cheese may help wipe out that stubborn abdominal fat, think again. This is a topic for another post. But, briefly, this study suggests that cheese consumption may help reduce visceral fat. Visceral fat, however, is generally fairly easy to mobilize (i.e., burn); much easier than the stubborn subcutaneous body fat that accumulates in the lower abdomen of middle-aged men and women. In middle-aged women, stubborn subcutaneous fat also accumulates in the hips and thighs.
Could eating Gouda cheese, together with other interventions (e.g., exercise), become a new weapon against the metabolic syndrome?
References:
Higurashi, S., Kunieda, Y., Matsuyama, H., & Kawakami, H. (2007). Effect of cheese consumption on the accumulation of abdominal adipose and decrease in serum adiponectin levels in rats fed a calorie dense diet. International Dairy Journal, 17(10), 1224–1231.
Walther, B., Schmid, A., Sieber, R., & Wehrmüller, K. (2008). Cheese in nutrition and health. Dairy Science Technology, 88(4), 389-405.
UPDATE on Pandemic Planning and Response
Between 23rd and 25th March 2010 I took part in the first Asia Europe Foundation Network for Public Health workshop exploring ways of enhancing the pandemic preparedness capabilities across partner countries. This, the first of three workshops, brought together 26 high-level participants from multiple sectors including amongst others, scientists, governments, NGO’s and the health sector. The event was facilitated by Prospex http://www.prospex.com/ and managed by the Asia Europe Foundation (ASEF) http://www.asef.org/
Although the detail of the work, is at this stage not public, I’d like to share some of the themes and process’ to keep those of you who are interested in this agenda, up to speed. I’d also like to encourage dialogue around these themes to continue our own explorations of this agenda.
Through an active participatory workshop the partners identified some of the driving factors and uncertainties around future pandemics and as such, were exploring the themes to feed into the second session which will develop and test some of the ideas which in turn, will feed the third session aiming to refine and analyze the scenarios developed to inform long-term strategic implementation and outreach.
The main elements of this first workshop involved participants identifying their own hopes and fears around the issues which included aspects of co-ordination, current preparedness and human capacity. The facilitators enabled the group to contextualise individual factors and further explore those from very specific perspectives ranging from the legal, economic and political, to demographic, ethical and cultural.
By introducing and interrogating existing foresight studies, the facilitators enabled, (for what was many of us our introduction to this field) an analysis of the successes and failings of contemporary thought. Much of the workshop unpicked the uncertainties associated with pandemic and enabled a clustering of factors with innovation (R&D) and notions of the Human Factor high on the agenda. Through the exploration of 15 clusters, the group further identified polarities in thinking and possibilities of response.
By way of example, I was personally very engaged in conversation around information and communication, particularly with reference to how messages are communicated. The polarities explored around these factors focused on whether pandemic messages would be critically received, or would be met with indifference. Worse than this perhaps, and a theme of many of my papers relating to arts/public health; would be that the media propagate hysteria. Participants from the media sector who took part in this work provided strong critical debate and crucial input into my thinking.
With hindsight, many of the subtle discussions I’d had here in Manchester prior to the workshop, weren’t given a full airing, but notions of the human element, universal metaphor and understanding the roles of both the media and new technologies were robustly discussed as part of the bigger picture. I was also able to make opportunities to discuss the notion of diversity and how different societies/communities around the world will interpret messages differently.
Whilst a good deal of the workshop gave opportunity for blue-sky thinking, it grounded very diverse perspectives in a community of joint interest. For my own part being jet-lagged, in a strange environment and in extreme heat made for quite an anxious start. However, the sessions were meticulously thought through and conducted in English. It is mortifying as a native English speaker, to be surrounded by people for whom this is a second or third language and who speak far more eloquently and with insight than someone who can barely master his own tongue. The company was truly diverse and I felt under a good deal of pressure as one of only two participants from the arts sector.
I most certainly feel that I added to the mix and injected the notion of thinking creatively around this public health agenda, but I could have more explicitly inputted on the role of the artist in society, both as reflecting and questioning societal norms. In particular, the role of the artist within research and development and community engagement is an area I hope to develop further in this work.
I was impressed by the creative insight of a number of participants from wildly different backgrounds, who positively exuded a deep understanding around the potency and relevance of the arts to this area of work.
Clive Parkinson
Although the detail of the work, is at this stage not public, I’d like to share some of the themes and process’ to keep those of you who are interested in this agenda, up to speed. I’d also like to encourage dialogue around these themes to continue our own explorations of this agenda.
Through an active participatory workshop the partners identified some of the driving factors and uncertainties around future pandemics and as such, were exploring the themes to feed into the second session which will develop and test some of the ideas which in turn, will feed the third session aiming to refine and analyze the scenarios developed to inform long-term strategic implementation and outreach.
The main elements of this first workshop involved participants identifying their own hopes and fears around the issues which included aspects of co-ordination, current preparedness and human capacity. The facilitators enabled the group to contextualise individual factors and further explore those from very specific perspectives ranging from the legal, economic and political, to demographic, ethical and cultural.
By introducing and interrogating existing foresight studies, the facilitators enabled, (for what was many of us our introduction to this field) an analysis of the successes and failings of contemporary thought. Much of the workshop unpicked the uncertainties associated with pandemic and enabled a clustering of factors with innovation (R&D) and notions of the Human Factor high on the agenda. Through the exploration of 15 clusters, the group further identified polarities in thinking and possibilities of response.
By way of example, I was personally very engaged in conversation around information and communication, particularly with reference to how messages are communicated. The polarities explored around these factors focused on whether pandemic messages would be critically received, or would be met with indifference. Worse than this perhaps, and a theme of many of my papers relating to arts/public health; would be that the media propagate hysteria. Participants from the media sector who took part in this work provided strong critical debate and crucial input into my thinking.
With hindsight, many of the subtle discussions I’d had here in Manchester prior to the workshop, weren’t given a full airing, but notions of the human element, universal metaphor and understanding the roles of both the media and new technologies were robustly discussed as part of the bigger picture. I was also able to make opportunities to discuss the notion of diversity and how different societies/communities around the world will interpret messages differently.
Whilst a good deal of the workshop gave opportunity for blue-sky thinking, it grounded very diverse perspectives in a community of joint interest. For my own part being jet-lagged, in a strange environment and in extreme heat made for quite an anxious start. However, the sessions were meticulously thought through and conducted in English. It is mortifying as a native English speaker, to be surrounded by people for whom this is a second or third language and who speak far more eloquently and with insight than someone who can barely master his own tongue. The company was truly diverse and I felt under a good deal of pressure as one of only two participants from the arts sector.
I most certainly feel that I added to the mix and injected the notion of thinking creatively around this public health agenda, but I could have more explicitly inputted on the role of the artist in society, both as reflecting and questioning societal norms. In particular, the role of the artist within research and development and community engagement is an area I hope to develop further in this work.
I was impressed by the creative insight of a number of participants from wildly different backgrounds, who positively exuded a deep understanding around the potency and relevance of the arts to this area of work.
Clive Parkinson
Sunday, May 16, 2010
How a Patient May Avoid An Angioplasty
Angioplasty is a procedure performed by inserting a catheter with a deflated balloon into an affected artery, then inflated to open the artery. Sometimes a stent, or mesh tube, is left to keep it open. The procedure does come with some risk, in fact, having the potential of inducing a heart attack.
If a patient is uncomfortable with an angioplasty, there are now other alternatives that may be just as effective without the procedure.
Medical researchers, for example, have been evaluating the combined approach using anti-coagulants, thrombolytic therapy (clot-dissolving drugs) and cholesterol-lowering drugs. According to Dr. Eric J. Topol of the Cleveland Clinic, the treatment has been deemed effective in at least a few small studies (1).
Other cardiologists look to intensive-lipid therapy alongside dietary supplements such as fish oil and vitamin D. According to Dr. William Davis, the integrated therapy has been shown to help slow progression of atherosclerosis and even reverse it in asymptomatic adults (2).
Along with treatment, the patient should adopt exercise and special dietary considerations to help provide a complete comprehensive treatment of risk factors including control of hypertension, obesity and type 2 diabetes (3). For this patient, diet should be low in saturated and trans fat, high in fiber, and provide optimal levels of nutrients such as omega-3 fatty acids, and vitamin D for lowering cardiovascular risk (3).
A DASH eating plan can help to meet diet goals. The eating plan, which has been found to lower blood pressure within 15 days, features low-fat dairy products, fish, and lean meats as well as plenty of whole grains, fruits and vegetables. Recently, a study found that a DASH eating plan combined with exercise helped subjects to reduce blood pressure, lose weight, improve mental function, and improve cardiovascular fitness (4).
References
1. Topol EJ. Integration of anticoagulation, thrombolysis and coronary angioplasty for unstable angina pectoris. Am J Cardiol. 1991 Sep 3;68(7):136B-141B.
2. Davis W, Rockway S, Kwasny M. Effect of a combined therapeutic approach of intensive lipid management, omega-3 fatty acid supplementation, and increased serum 25 (OH) vitamin D on coronary calcium scores in asymptomatic adults. Am J Ther. 2009 Jul-Aug;16(4):326-32. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19092644
3. Kohlstadt I. Food and Nutrients in Disease Management. Boca Raton, FL: CRC Press, 2009.
4. Smith PJ, Blumenthal JA, Babyak MA, Craighead L, Welsh-Bohmer KA, Browndyke JN, Strauman TA, Sherwood A. Effects of the Dietary Approaches to Stop Hypertension Diet, Exercise, and Caloric Restriction on Neurocognition in Overweight Adults With High Blood Pressure. Hypertension. 2010 Mar 19. [Epub ahead of print]
If a patient is uncomfortable with an angioplasty, there are now other alternatives that may be just as effective without the procedure.
Medical researchers, for example, have been evaluating the combined approach using anti-coagulants, thrombolytic therapy (clot-dissolving drugs) and cholesterol-lowering drugs. According to Dr. Eric J. Topol of the Cleveland Clinic, the treatment has been deemed effective in at least a few small studies (1).
Other cardiologists look to intensive-lipid therapy alongside dietary supplements such as fish oil and vitamin D. According to Dr. William Davis, the integrated therapy has been shown to help slow progression of atherosclerosis and even reverse it in asymptomatic adults (2).
Along with treatment, the patient should adopt exercise and special dietary considerations to help provide a complete comprehensive treatment of risk factors including control of hypertension, obesity and type 2 diabetes (3). For this patient, diet should be low in saturated and trans fat, high in fiber, and provide optimal levels of nutrients such as omega-3 fatty acids, and vitamin D for lowering cardiovascular risk (3).
A DASH eating plan can help to meet diet goals. The eating plan, which has been found to lower blood pressure within 15 days, features low-fat dairy products, fish, and lean meats as well as plenty of whole grains, fruits and vegetables. Recently, a study found that a DASH eating plan combined with exercise helped subjects to reduce blood pressure, lose weight, improve mental function, and improve cardiovascular fitness (4).
References
1. Topol EJ. Integration of anticoagulation, thrombolysis and coronary angioplasty for unstable angina pectoris. Am J Cardiol. 1991 Sep 3;68(7):136B-141B.
2. Davis W, Rockway S, Kwasny M. Effect of a combined therapeutic approach of intensive lipid management, omega-3 fatty acid supplementation, and increased serum 25 (OH) vitamin D on coronary calcium scores in asymptomatic adults. Am J Ther. 2009 Jul-Aug;16(4):326-32. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19092644
3. Kohlstadt I. Food and Nutrients in Disease Management. Boca Raton, FL: CRC Press, 2009.
4. Smith PJ, Blumenthal JA, Babyak MA, Craighead L, Welsh-Bohmer KA, Browndyke JN, Strauman TA, Sherwood A. Effects of the Dietary Approaches to Stop Hypertension Diet, Exercise, and Caloric Restriction on Neurocognition in Overweight Adults With High Blood Pressure. Hypertension. 2010 Mar 19. [Epub ahead of print]
Labels:
nutr therap
Saturday, May 15, 2010
Intermittent fasting as a form of liberation
I have been doing a lot of reading over the years on isolated hunter-gatherer populations; see three references at the end of this post, all superb sources (Chagnon’s book on the Yanomamo, in particular, is an absolute page turner). I also take every opportunity I have to talk with anthropologists and other researchers who have had field experience with hunter-gatherer groups. Even yesterday I was talking to a researcher who spent many years living among isolated native Brazilian groups in the Amazon.
Maybe I have been reading too much into those descriptions, but it seems to me that one distinctive feature of many adults in hunter-gatherer populations, when compared with adults in urban populations, is that the hunter-gatherers are a lot less obsessed with food.
Interestingly, this seems to be a common characteristic of physically active children. They want to play, and eating is often an afterthought, an interruption of play. Sedentary children, who play indoors, can and often want to eat while they play.
Perhaps adult hunter-gatherers are more like physically active children than adults in modern urban societies. Maybe this is one of the reasons why adult hunter-gatherers have much less body fat. Take a look at the photo below (click to enlarge), from Wikipedia. It was reportedly taken in 1939, and shows three Australian aboriginals.
Hunter-gatherers do not have supermarkets, and active children need food to grow healthy. Adult urbanites have easy access to an abundance of food in supermarkets, and they do not need food to grow, at least not vertically.
Still, adult hunter-gatherers and children who are physically active are generally much less concerned about food than adults in modern urban societies.
It seems illogical, a bit like a mental disorder of some sort that has been plaguing adults in modern urban societies. A mental disorder that contributes to making them obese.
Modern urbanites are constantly worried about food. And also about material possessions, bills, taxes etc. They want to accumulate as much wealth as their personal circumstances allow them, so that they can retire and pay for medical expenses. They must worry about paying for their children’s education. Food is one of their many worries; for many it is the biggest of them all. Too much food makes you fat, too little makes you lose muscle (not really true, but a widespread belief).
Generally speaking, intermittent fasting is very good for human health. Humans seem to have evolved to be episodic eaters, being in the fasted state most of the time. This is perhaps why intermittent fasting significantly reduces levels of inflammation markers, promotes the recycling of “messed up” proteins (e.g., glycated proteins), and increases leptin and insulin sensitivity. It is something natural. I am talking about fasting 24 h at a time (or a bit more, but not much more than that), with plenty of water but no calories. Even skipping a meal now and then, when you are busy with other things, is a form of intermittent fasting.
Now, the idea that our hominid ancestors were starving most of the time does not make a lot of sense, at least not when we think about Homo sapiens, as opposed to earlier ancestors (e.g., the Australopithecines). Even archaic Homo sapiens, dating back to 500 thousand years ago, were probably too smart to be constantly starving. Moreover, the African savannas, where Homo sapiens emerged, were not the type of environment where a smart and social species would be hungry for too long.
Yet, intermittent fasting probably happened frequently among our Homo sapiens ancestors, for the same reason that it happens among hunter-gatherers and active children today. My guess is that, by and large, our ancestors were simply not too worried about food. They ate it because they were hungry, probably at regular times – as most hunter-gatherers do. They skipped meals from time to time.
They certainly did not eat to increase their metabolism, raise their thyroid hormone levels, or have a balanced macronutrient intake.
There were no doubt special occasions when people gathered for a meal as a social activity, but probably the focus was on the social activity, and secondarily on the food.
Of course, they did not have doughnuts around, or foods engineered to make people addicted to them. That probably made things a little easier.
Successful body fat loss through intermittent fasting requires a change in mindset.
References:
Boaz, N.T., & Almquist, A.J. (2001). Biological anthropology: A synthetic approach to human evolution. Upper Saddle River, NJ: Prentice Hall.
Chagnon, N.A. (1977). Yanomamo: The fierce people. New York, NY: Holt, Rinehart and Winston.
Price, W.A. (2008). Nutrition and physical degeneration. La Mesa, CA: Price-Pottenger Nutrition Foundation.
Maybe I have been reading too much into those descriptions, but it seems to me that one distinctive feature of many adults in hunter-gatherer populations, when compared with adults in urban populations, is that the hunter-gatherers are a lot less obsessed with food.
Interestingly, this seems to be a common characteristic of physically active children. They want to play, and eating is often an afterthought, an interruption of play. Sedentary children, who play indoors, can and often want to eat while they play.
Perhaps adult hunter-gatherers are more like physically active children than adults in modern urban societies. Maybe this is one of the reasons why adult hunter-gatherers have much less body fat. Take a look at the photo below (click to enlarge), from Wikipedia. It was reportedly taken in 1939, and shows three Australian aboriginals.
Hunter-gatherers do not have supermarkets, and active children need food to grow healthy. Adult urbanites have easy access to an abundance of food in supermarkets, and they do not need food to grow, at least not vertically.
Still, adult hunter-gatherers and children who are physically active are generally much less concerned about food than adults in modern urban societies.
It seems illogical, a bit like a mental disorder of some sort that has been plaguing adults in modern urban societies. A mental disorder that contributes to making them obese.
Modern urbanites are constantly worried about food. And also about material possessions, bills, taxes etc. They want to accumulate as much wealth as their personal circumstances allow them, so that they can retire and pay for medical expenses. They must worry about paying for their children’s education. Food is one of their many worries; for many it is the biggest of them all. Too much food makes you fat, too little makes you lose muscle (not really true, but a widespread belief).
Generally speaking, intermittent fasting is very good for human health. Humans seem to have evolved to be episodic eaters, being in the fasted state most of the time. This is perhaps why intermittent fasting significantly reduces levels of inflammation markers, promotes the recycling of “messed up” proteins (e.g., glycated proteins), and increases leptin and insulin sensitivity. It is something natural. I am talking about fasting 24 h at a time (or a bit more, but not much more than that), with plenty of water but no calories. Even skipping a meal now and then, when you are busy with other things, is a form of intermittent fasting.
Now, the idea that our hominid ancestors were starving most of the time does not make a lot of sense, at least not when we think about Homo sapiens, as opposed to earlier ancestors (e.g., the Australopithecines). Even archaic Homo sapiens, dating back to 500 thousand years ago, were probably too smart to be constantly starving. Moreover, the African savannas, where Homo sapiens emerged, were not the type of environment where a smart and social species would be hungry for too long.
Yet, intermittent fasting probably happened frequently among our Homo sapiens ancestors, for the same reason that it happens among hunter-gatherers and active children today. My guess is that, by and large, our ancestors were simply not too worried about food. They ate it because they were hungry, probably at regular times – as most hunter-gatherers do. They skipped meals from time to time.
They certainly did not eat to increase their metabolism, raise their thyroid hormone levels, or have a balanced macronutrient intake.
There were no doubt special occasions when people gathered for a meal as a social activity, but probably the focus was on the social activity, and secondarily on the food.
Of course, they did not have doughnuts around, or foods engineered to make people addicted to them. That probably made things a little easier.
Successful body fat loss through intermittent fasting requires a change in mindset.
References:
Boaz, N.T., & Almquist, A.J. (2001). Biological anthropology: A synthetic approach to human evolution. Upper Saddle River, NJ: Prentice Hall.
Chagnon, N.A. (1977). Yanomamo: The fierce people. New York, NY: Holt, Rinehart and Winston.
Price, W.A. (2008). Nutrition and physical degeneration. La Mesa, CA: Price-Pottenger Nutrition Foundation.
Friday, May 14, 2010
Atherosclerosis
Atherosclerosis refers to accumulation of a thick sludge in patches that merge to form large plaques, called atheromas, in artery walls. The plaque is made up of cholesterol and other fats, macrophages, cell "junk", calcium, and tissues.
LDL cholesterol is associated with atherogenesis because as it becomes oxidized it can induce endothelial cells to attract blood-borne monycytes, transforming them into macrophages and trapping them in endothelial spaces (1).
The macrophages then engorge themselves with cholesterol and fat creating "foam cells. Then, once engorged, they release inflammatory cytokines that only lead to even more macrophages creating more foam cells (1).
Along with damaged smooth muscle cells, the foam cells then form the sludge plaque, or fatty streak, that narrows lumen as it grows larger causing blood flow to to become restricted (1).
Medications
There are various drugs that can help to slow or reverse atherosclerosis, which include cholesterol-lowering drugs such as statins, anti-coagulants such as warfarin to inhibit clotting, antiplatelets like aspirin to keep platelets from forming clots, and medications such as ACE inhibitors or calcium channel blockers to lower blood pressure (2).
If atherosclerosis becomes severe, surgery may be needed. A procedure called an angioplasty can be performed by inserting a catheter with a deflated balloon into an affected artery, then inflated to open the artery. Sometimes a stent, or mesh tube, is left to keep it open.
Other surgeries involve endarterectomy, where fatty deposits are surgically removed from walls, or thrombolytic therapy in which drugs are inserted into arteries to dissolve clots (2).
A bypass surgery (such as a CABG, coronary artery bypass surgery) involves using another part of the body or a tube to allow blood to flow around an affected artery (2).
Lifestyle changes
It is possible to change the course of atherosclerosis -- even possibly reverse it -- by adopting a few lifestyle changes. These include stopping smoking, exercising regularly, eating right and lowering stress.
- Smoking in itself oxidizes LDL cholesterol and hastens the damage of arteries.
- Exercise improves blood flow and can induce the development of new blood vessels to lower the pressure on affected arteries.
- Eating right should include adopting strategies such as managing portions for weight management, a DASH-style diet for lowering blood pressure, limiting saturated and trans fatty acids and adopting polyunsaturated fats to lower triglycerides, and eating a high-fiber diet to lower cholesterol levels (1).
- Limiting stress in life through relaxation and sleeping well helps to avoid rises in blood pressure.
Overall, it's very likely that almost half of us will die from atherosclerosis or complications relating to it. Almost all of us have fatty streaks and plaques already developing. These are disheartening figures for those of us who wish to do all we can to fight back.
Luckily, our nutrition and medical knowledge continues to improve and new technologies are also forming.
One program of interest is the one promoted by cardiologist Dr. William Davis in his book Track Your Plaque, who promotes actively "tracking" the progression of plaque development (3).
In addition, Dr. Davis and fellow scientists have studied the effects of combined therapies involving niacin or statins, fish oil, vitamin D and other means to slow or reverse "hardening of the arteries" (4).
Reference List
1. Gropper SS, Smith JL, Groff JL. Advanced Nutrition and Human Metabolism. Belmont, CA: Thomson Wadsworth, 2009.
2. http://www.mayoclinic.com/health/arteriosclerosis-atherosclerosis/DS00525/DSECTION=treatments%2Dand%2Ddrugs
3. http://www.trackyourplaque.com
4. Davis W, Rockway S, Kwasny M. Effect of a combined therapeutic approach of intensive lipid management, omega-3 fatty acid supplementation, and increased serum 25 (OH) vitamin D on coronary calcium scores in asymptomatic adults. Am J Ther. 2009 Jul-Aug;16(4):326-32. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19092644
LDL cholesterol is associated with atherogenesis because as it becomes oxidized it can induce endothelial cells to attract blood-borne monycytes, transforming them into macrophages and trapping them in endothelial spaces (1).
The macrophages then engorge themselves with cholesterol and fat creating "foam cells. Then, once engorged, they release inflammatory cytokines that only lead to even more macrophages creating more foam cells (1).
Along with damaged smooth muscle cells, the foam cells then form the sludge plaque, or fatty streak, that narrows lumen as it grows larger causing blood flow to to become restricted (1).
Medications
There are various drugs that can help to slow or reverse atherosclerosis, which include cholesterol-lowering drugs such as statins, anti-coagulants such as warfarin to inhibit clotting, antiplatelets like aspirin to keep platelets from forming clots, and medications such as ACE inhibitors or calcium channel blockers to lower blood pressure (2).
If atherosclerosis becomes severe, surgery may be needed. A procedure called an angioplasty can be performed by inserting a catheter with a deflated balloon into an affected artery, then inflated to open the artery. Sometimes a stent, or mesh tube, is left to keep it open.
Other surgeries involve endarterectomy, where fatty deposits are surgically removed from walls, or thrombolytic therapy in which drugs are inserted into arteries to dissolve clots (2).
A bypass surgery (such as a CABG, coronary artery bypass surgery) involves using another part of the body or a tube to allow blood to flow around an affected artery (2).
Lifestyle changes
It is possible to change the course of atherosclerosis -- even possibly reverse it -- by adopting a few lifestyle changes. These include stopping smoking, exercising regularly, eating right and lowering stress.
- Smoking in itself oxidizes LDL cholesterol and hastens the damage of arteries.
- Exercise improves blood flow and can induce the development of new blood vessels to lower the pressure on affected arteries.
- Eating right should include adopting strategies such as managing portions for weight management, a DASH-style diet for lowering blood pressure, limiting saturated and trans fatty acids and adopting polyunsaturated fats to lower triglycerides, and eating a high-fiber diet to lower cholesterol levels (1).
- Limiting stress in life through relaxation and sleeping well helps to avoid rises in blood pressure.
Overall, it's very likely that almost half of us will die from atherosclerosis or complications relating to it. Almost all of us have fatty streaks and plaques already developing. These are disheartening figures for those of us who wish to do all we can to fight back.
Luckily, our nutrition and medical knowledge continues to improve and new technologies are also forming.
One program of interest is the one promoted by cardiologist Dr. William Davis in his book Track Your Plaque, who promotes actively "tracking" the progression of plaque development (3).
In addition, Dr. Davis and fellow scientists have studied the effects of combined therapies involving niacin or statins, fish oil, vitamin D and other means to slow or reverse "hardening of the arteries" (4).
Reference List
1. Gropper SS, Smith JL, Groff JL. Advanced Nutrition and Human Metabolism. Belmont, CA: Thomson Wadsworth, 2009.
2. http://www.mayoclinic.com/health/arteriosclerosis-atherosclerosis/DS00525/DSECTION=treatments%2Dand%2Ddrugs
3. http://www.trackyourplaque.com
4. Davis W, Rockway S, Kwasny M. Effect of a combined therapeutic approach of intensive lipid management, omega-3 fatty acid supplementation, and increased serum 25 (OH) vitamin D on coronary calcium scores in asymptomatic adults. Am J Ther. 2009 Jul-Aug;16(4):326-32. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19092644
Wednesday, May 12, 2010
Is heavy physical activity a major trigger of death by sudden cardiac arrest? Not in Oregon
The idea that heavy physical activity is a main trigger of heart attacks is widespread. Often endurance running and cardio-type activities are singled out. Some people refer to this as “death by running”.
Good cardiology textbooks, such as the Mayo Clinic Cardiology, tend to give us a more complex and complete picture. So do medical research articles that report on studies of heart attacks based on comprehensive surveys.
Reddy and colleagues (2009) studied sudden cardiac arrest events followed by death from 2002 to 2005 in Multnomah County in Oregon. This study was part of the ongoing Oregon Sudden Unexpected Death Study. Multnomah County has an area of 435 square miles, and had a population of over 677 thousand at the time of the study. The full reference to the article and a link to a full-text version are at the end of this post.
The researchers grouped deaths by sudden cardiac arrests (SCAs) according to the main type of activity being performed before the event. Below is how the authors defined the activities, quoted verbatim from the article. MET is a measure of the amount of energy spent in the activity; one MET is the amount of energy spent by a person sitting quietly.
- Sleep (MET 0.9): subjects who were sleeping when they sustained SCA.
- Light activity (MET 1.0–3.4): included bathing, dressing, cooking, cleaning, feeding, household walking and driving.
- Moderate activity (MET 3.5–5.9): included walking for exercise, mowing lawn, gardening, working in the yard, dancing.
- Heavy activity (MET score ≥6): included sports such as tennis, running, jogging, treadmill, skiing, biking.
- Sexual activity (MET score 1.3): included acts of sexual intercourse.
What did they find? Not what many people would expect.
The vast majority of the people dying of sudden cardiac arrest were doing things that fit the “light activity” group above prior to their death. This applies to both genders. The figure below (click to enlarge) shows the percentages of men and women who died from sudden cardiac arrest, grouped by activity type.
Sudden cardiac arrests were also categorized as witnessed or un-witnessed. For witnessed, someone saw them happening. For un-witnessed, the person was seen alive, and within 24 hours had died. So the data for witnessed sudden cardiac arrests is a bit more reliable. The table below displays the distribution of mean age, gender and known coronary artery disease (CAD) in those with witnessed sudden cardiac arrest.
Look at the bottom row, showing those with known coronary artery disease. Again, light activity is the main trigger. Sleep comes second. The numbers within parentheses refer to percentages within each activity group. Those percentages are not very helpful in the identification of the most important triggers, although they do suggest that coronary artery disease is a major risk factor. For example, among those who died from sudden cardiac arrest while having sex, 57 percent had known coronary artery disease. For light activity, 36 percent had known coronary artery disease.
As a caveat, it is worth noting that heavy activity appears to be more of a trigger in younger individuals than in older ones. This may simply reflect the patterns of activities at different ages. However, this does not seem to properly account for the large differences observed in triggers; the standard deviation for age in the heavy activity group was large enough to include plenty of seniors. Still, it would have been nice to see a multivariate analysis controlling for various effects, including age.
So what is going on here?
The authors give us a hint. The real culprit may be bottled up emotional stress and sleep disorders; the latter may be caused by stress, as well as by obesity and other related problems. They have some data that points in those directions. That makes some sense.
We humans have evolved “fight-or-flight” mechanisms that involve large hormonal discharges in response to stressors. Our ancestors needed those. For example, they needed those to either fight or run for their lives in response to animal attacks.
Modern humans experience too many stressors while sitting down, as in stressful car commutes and nasty online interactions. The stresses cause “fight-or-flight” hormonal discharges, but are followed by neither “fight” nor “flight” in most cases. This cannot be very good for us.
Death by running!? More like death by not running!
Reference:
Reddy, P.R., Reinier, K., Singh, T., Mariani, R., Gunson, K., Jui, J., & Chugh, S.S. (2009). Physical activity as a trigger of sudden cardiac arrest: The Oregon Sudden Unexpected Death Study. International Journal of Cardiology, 131(3), 345–349.
Good cardiology textbooks, such as the Mayo Clinic Cardiology, tend to give us a more complex and complete picture. So do medical research articles that report on studies of heart attacks based on comprehensive surveys.
Reddy and colleagues (2009) studied sudden cardiac arrest events followed by death from 2002 to 2005 in Multnomah County in Oregon. This study was part of the ongoing Oregon Sudden Unexpected Death Study. Multnomah County has an area of 435 square miles, and had a population of over 677 thousand at the time of the study. The full reference to the article and a link to a full-text version are at the end of this post.
The researchers grouped deaths by sudden cardiac arrests (SCAs) according to the main type of activity being performed before the event. Below is how the authors defined the activities, quoted verbatim from the article. MET is a measure of the amount of energy spent in the activity; one MET is the amount of energy spent by a person sitting quietly.
- Sleep (MET 0.9): subjects who were sleeping when they sustained SCA.
- Light activity (MET 1.0–3.4): included bathing, dressing, cooking, cleaning, feeding, household walking and driving.
- Moderate activity (MET 3.5–5.9): included walking for exercise, mowing lawn, gardening, working in the yard, dancing.
- Heavy activity (MET score ≥6): included sports such as tennis, running, jogging, treadmill, skiing, biking.
- Sexual activity (MET score 1.3): included acts of sexual intercourse.
What did they find? Not what many people would expect.
The vast majority of the people dying of sudden cardiac arrest were doing things that fit the “light activity” group above prior to their death. This applies to both genders. The figure below (click to enlarge) shows the percentages of men and women who died from sudden cardiac arrest, grouped by activity type.
Sudden cardiac arrests were also categorized as witnessed or un-witnessed. For witnessed, someone saw them happening. For un-witnessed, the person was seen alive, and within 24 hours had died. So the data for witnessed sudden cardiac arrests is a bit more reliable. The table below displays the distribution of mean age, gender and known coronary artery disease (CAD) in those with witnessed sudden cardiac arrest.
Look at the bottom row, showing those with known coronary artery disease. Again, light activity is the main trigger. Sleep comes second. The numbers within parentheses refer to percentages within each activity group. Those percentages are not very helpful in the identification of the most important triggers, although they do suggest that coronary artery disease is a major risk factor. For example, among those who died from sudden cardiac arrest while having sex, 57 percent had known coronary artery disease. For light activity, 36 percent had known coronary artery disease.
As a caveat, it is worth noting that heavy activity appears to be more of a trigger in younger individuals than in older ones. This may simply reflect the patterns of activities at different ages. However, this does not seem to properly account for the large differences observed in triggers; the standard deviation for age in the heavy activity group was large enough to include plenty of seniors. Still, it would have been nice to see a multivariate analysis controlling for various effects, including age.
So what is going on here?
The authors give us a hint. The real culprit may be bottled up emotional stress and sleep disorders; the latter may be caused by stress, as well as by obesity and other related problems. They have some data that points in those directions. That makes some sense.
We humans have evolved “fight-or-flight” mechanisms that involve large hormonal discharges in response to stressors. Our ancestors needed those. For example, they needed those to either fight or run for their lives in response to animal attacks.
Modern humans experience too many stressors while sitting down, as in stressful car commutes and nasty online interactions. The stresses cause “fight-or-flight” hormonal discharges, but are followed by neither “fight” nor “flight” in most cases. This cannot be very good for us.
Death by running!? More like death by not running!
Reference:
Reddy, P.R., Reinier, K., Singh, T., Mariani, R., Gunson, K., Jui, J., & Chugh, S.S. (2009). Physical activity as a trigger of sudden cardiac arrest: The Oregon Sudden Unexpected Death Study. International Journal of Cardiology, 131(3), 345–349.
Sunday, May 9, 2010
Sodium: How much is right for you?
Sodium’s association with high blood pressure is well known. However, sodium also plays a large role in keeping you healthy. It’s important to know how to strike the right balance.
Along with potassium, sodium is essential for fluid balance, facilitating the flow of water in and out of cells to bring nutrients in and take wastes away. Sodium also has a role in the regulation of blood pressure and helping muscles and the heart relax. Each sodium ion contains an electrical charge, acting as an electrolyte, which allows transmission of nerve impulses to the brain and throughout the body.
Sodium levels in the body are controlled by the kidneys. If the body doesn’t receive enough sodium daily—a chronic problem for our early ancestors—then the kidneys retain sodium. When the body has a high enough amount, then the excess sodium is excreted in the urine.
At times, sodium levels may fluctuate. If a person has a dysfunctional kidney, then the body may retain too much sodium, which can result in edema, or swelling in the legs and feet because sodium attracts water. In contrast, diarrhea or vomiting may result decreased sodium levels, a condition known as hyponatremia.
How sodium regulates blood pressure is not entirely understood, but there is an established link between high sodium intake and high blood pressure. As expected, there is also a link between sodium reduction and lower blood pressure.
The sodium-hypertension relationship may also have to do with how sodium interplays with other minerals such as potassium and calcium. Potassium, for example, appears to assist the kidneys in shedding excess sodium. Lowering sodium intake also helps to conserve calcium, which may affect blood pressure.
Recommendations for Sodium
The Institute of Medicine is recommending an Adequate Intake of sodium at 1,500 mg per day for adults and children 9-13 as well as 1,000 mg and 1,200 mg per day for children ages 1-3 and 4-8, respectively. These levels are considered appropriate for replacing daily losses via sweat and urine. The need for sodium may be slightly greater if exercise produces excessive sweating or if a person has symptoms of vomiting or diarrhea.
On average, however, most adults in the U.S. consume about 3,200 milligrams or more a day. With these figures, it is easy to understand why high blood pressure affects nearly 75 million Americans. The average intake is well above the Institute of Medicine’s Tolerable Upper Intake Level of 2,300 milligrams per day for adults and 1,500mg, 1,900mg and 2,200 mg for children ages 1-3, 4-8 and 9-13, respectively.
Cutting sodium intake daily tor recommended levels is important and it doesn’t have to be difficult with these three simple strategies:
Sodium Strategy #1: Limit processed or prepared foods high in sodium. Most sodium in the diet doesn’t come from the salt shaker, but from processed and prepared foods. Thus, the best way to lower sodium is to reduce intake of processed foods or replace them with low-sodium alternatives. This includes ready-to-eat packaged foods such as potato chips, fast-food meals such as burritos, and highly salted meals prepared at restaurants.
Sodium Strategy #2: Learn to enjoy food without salt. Taste food before salting it; the food may already be salty enough or it may be enjoyed without salt. In fact, salty is an acquired taste. The body and taste buds can easily adjust to less salt. Studies have shown that as people reduce salt intake and stick to a relatively lower intake of sodium, they will naturally begin to prefer foods with less salt. When eating at home, try not having the salt shaker on the table and, if eating out, simply move salt shakers to another table. When preparing food, try using less salt and seasoning food with spices or salt-substitutes instead. Keep an eye on store-bought spice blends, though, as many may contain high amounts of salt.
Sodium Strategy #3: Balance sodium with potassium-rich fruits and vegetables. A clear association exists between higher potassium intake from fruits and vegetables and lower blood pressure regardless of sodium intake. Potassium helps the kidneys in promoting sodium excretion, reduces urinary calcium and magnesium (which influence blood pressure), supports smooth vascular muscle health, and helps with regulation of blood pressure.
Less Sodium in a DASH
Most people who are interested in maintaining healthy blood pressure levels would do best to follow a DASH (Dietary Approaches to Stop Hypertension)eating plan. In the well-known DASH-sodium study, which was conducted by the National Heart, Lung and Blood Institute, people following the diet lowered blood pressure in just 14 days even without reducing salt intake.
The DASH eating plan includes consuming a diet rich in low-fat, low-sodium dairy products, fish, chicken and lean meats as well as large amount of whole grains, fruits and vegetables.
When a person is concerned about blood pressure, the best advice nutritionists can give is to begin following a DASH eating plan combined with regular exercise and weight management. In fact, according to a recent study in Hypertension, this plan helped people reduce blood pressure, lose weight, improve mental function, and improve cardiovascular health.
Taking the Pressure Off of Sodium
It’s extremely easy to place all of the blame for society’s high blood pressure woes and medical costs on sodium, but the mineral’s role in the body should not be ignored. Sodium is essential for good health and too little could lead to other health issues, including deficiencies in iodine, which is mainly provided in the North American diet from iodized salt.
While lowering sodium consumption can lead to a natural preference for foods with less salt, it’s important not to cut salt out completely. Because the body requires some sodium to function properly, avoiding salt entirely might backfire, and cause cravings for high-sodium foods. As with almost all vitamins and minerals, the key to healthy sodium intake is always balance with other nutrients. A DASH eating plan and strategies for maintaining a healthy intake (such as those given above) can help you achieve this balance of nutrients for healthy blood pressure levels and optimal health.
Reference
Dyuff RL, American Dietitic Association. American Dietetic Association Complete Food and Nutrition Guide, 3rd edition. 2006. Wiley.
Along with potassium, sodium is essential for fluid balance, facilitating the flow of water in and out of cells to bring nutrients in and take wastes away. Sodium also has a role in the regulation of blood pressure and helping muscles and the heart relax. Each sodium ion contains an electrical charge, acting as an electrolyte, which allows transmission of nerve impulses to the brain and throughout the body.
Sodium levels in the body are controlled by the kidneys. If the body doesn’t receive enough sodium daily—a chronic problem for our early ancestors—then the kidneys retain sodium. When the body has a high enough amount, then the excess sodium is excreted in the urine.
At times, sodium levels may fluctuate. If a person has a dysfunctional kidney, then the body may retain too much sodium, which can result in edema, or swelling in the legs and feet because sodium attracts water. In contrast, diarrhea or vomiting may result decreased sodium levels, a condition known as hyponatremia.
How sodium regulates blood pressure is not entirely understood, but there is an established link between high sodium intake and high blood pressure. As expected, there is also a link between sodium reduction and lower blood pressure.
The sodium-hypertension relationship may also have to do with how sodium interplays with other minerals such as potassium and calcium. Potassium, for example, appears to assist the kidneys in shedding excess sodium. Lowering sodium intake also helps to conserve calcium, which may affect blood pressure.
Recommendations for Sodium
The Institute of Medicine is recommending an Adequate Intake of sodium at 1,500 mg per day for adults and children 9-13 as well as 1,000 mg and 1,200 mg per day for children ages 1-3 and 4-8, respectively. These levels are considered appropriate for replacing daily losses via sweat and urine. The need for sodium may be slightly greater if exercise produces excessive sweating or if a person has symptoms of vomiting or diarrhea.
On average, however, most adults in the U.S. consume about 3,200 milligrams or more a day. With these figures, it is easy to understand why high blood pressure affects nearly 75 million Americans. The average intake is well above the Institute of Medicine’s Tolerable Upper Intake Level of 2,300 milligrams per day for adults and 1,500mg, 1,900mg and 2,200 mg for children ages 1-3, 4-8 and 9-13, respectively.
Cutting sodium intake daily tor recommended levels is important and it doesn’t have to be difficult with these three simple strategies:
Sodium Strategy #1: Limit processed or prepared foods high in sodium. Most sodium in the diet doesn’t come from the salt shaker, but from processed and prepared foods. Thus, the best way to lower sodium is to reduce intake of processed foods or replace them with low-sodium alternatives. This includes ready-to-eat packaged foods such as potato chips, fast-food meals such as burritos, and highly salted meals prepared at restaurants.
Sodium Strategy #2: Learn to enjoy food without salt. Taste food before salting it; the food may already be salty enough or it may be enjoyed without salt. In fact, salty is an acquired taste. The body and taste buds can easily adjust to less salt. Studies have shown that as people reduce salt intake and stick to a relatively lower intake of sodium, they will naturally begin to prefer foods with less salt. When eating at home, try not having the salt shaker on the table and, if eating out, simply move salt shakers to another table. When preparing food, try using less salt and seasoning food with spices or salt-substitutes instead. Keep an eye on store-bought spice blends, though, as many may contain high amounts of salt.
Sodium Strategy #3: Balance sodium with potassium-rich fruits and vegetables. A clear association exists between higher potassium intake from fruits and vegetables and lower blood pressure regardless of sodium intake. Potassium helps the kidneys in promoting sodium excretion, reduces urinary calcium and magnesium (which influence blood pressure), supports smooth vascular muscle health, and helps with regulation of blood pressure.
Less Sodium in a DASH
Most people who are interested in maintaining healthy blood pressure levels would do best to follow a DASH (Dietary Approaches to Stop Hypertension)eating plan. In the well-known DASH-sodium study, which was conducted by the National Heart, Lung and Blood Institute, people following the diet lowered blood pressure in just 14 days even without reducing salt intake.
The DASH eating plan includes consuming a diet rich in low-fat, low-sodium dairy products, fish, chicken and lean meats as well as large amount of whole grains, fruits and vegetables.
When a person is concerned about blood pressure, the best advice nutritionists can give is to begin following a DASH eating plan combined with regular exercise and weight management. In fact, according to a recent study in Hypertension, this plan helped people reduce blood pressure, lose weight, improve mental function, and improve cardiovascular health.
Taking the Pressure Off of Sodium
It’s extremely easy to place all of the blame for society’s high blood pressure woes and medical costs on sodium, but the mineral’s role in the body should not be ignored. Sodium is essential for good health and too little could lead to other health issues, including deficiencies in iodine, which is mainly provided in the North American diet from iodized salt.
While lowering sodium consumption can lead to a natural preference for foods with less salt, it’s important not to cut salt out completely. Because the body requires some sodium to function properly, avoiding salt entirely might backfire, and cause cravings for high-sodium foods. As with almost all vitamins and minerals, the key to healthy sodium intake is always balance with other nutrients. A DASH eating plan and strategies for maintaining a healthy intake (such as those given above) can help you achieve this balance of nutrients for healthy blood pressure levels and optimal health.
Reference
Dyuff RL, American Dietitic Association. American Dietetic Association Complete Food and Nutrition Guide, 3rd edition. 2006. Wiley.
More reason to love olive oil
I use one particular olive oil for cooking and another extra-virgin olive oil to mix with some balsamic vinegar for my salads. Olive oil, as the staple source of fatty acids in the Mediterranean diet, has also been heavily researched for its health benefits especially in comparison to other sources of fat such as butter, corn or soy oil.
On April 20, a study in BMC Genomics was published that found that olive oil eaten at breakfast modified gene expression in patients with metabolic syndrome (1). The breakfast caused the changes in mononuclear cells after intake of the olive oil and repressed pro-inflammatory genes (1).
The study was performed on 20 patients in a double-blind randomized trial (1). The researchers noted that many of the genes were also implicated in type 2 diabetes, dyslipidemia and obesity (1).
The study adds to evidence that olive oil helps reduce inflammation unlike other oils such as butter (2) and, thereby, adds to the reasons why the Mediterranean diet is associated with lower risk of cardiovascular disease (3).
References
1. Camargo A, Ruano J, Fernandez JM, Parnell LD, Jimenez A, Santos-Gonzalez M, Marin C, Perez-Martinez P, Uceda M, Lopez-Miranda J, Perez-Jimenez F. Gene expression changes in mononuclear cells from patients with metabolic syndrome after acute intake of phenol-rich virgin olive oil. BMC Genomics. 2010 Apr 20;11(1):253. [Epub ahead of print]
2. Bellido C, López-Miranda J, Blanco-Colio LM, Pérez-Martínez P, Muriana FJ, Martín-Ventura JL, Marín C, Gómez P, Fuentes F, Egido J, Pérez-Jiménez F. Butter and walnuts, but not olive oil, elicit postprandial activation of nuclear transcription factor kappaB in peripheral blood mononuclear cells from healthy men.Am J Clin Nutr. 2004 Dec;80(6):1487-91.
3. Bellido C. Perez-Jimenez F, Alvarez de Cienfuegos G, Badimon L, Barja G, Battino M, Blanco A, Bonanome A, Colomer R, Corella-Piquer D, Covas I, Chamorro-Quiros J, Escrich E, Gaforio JJ, Garcia Luna PP, Hidalgo L, Kafatos A, Kris-Etherton PM, Lairon D, Lamuela-Raventos R, Lopez-Miranda J, Lopez-Segura F, Martinez-Gonzalez MA, Mata P, Mataix J, Ordovas J, Osada J, Pacheco-Reyes R, Perucho M, Pineda-Priego M, Quiles JL, Ramirez-Tortosa MC, Ruiz-Gutierrez V, Sanchez-Rovira P, Solfrizzi V, Soriguer-Escofet F, de la Torre-Fornell R, Trichopoulos A, Villalba-Montoro JM, Villar-Ortiz JR, Visioli F. International conference on the healthy effect of virgin olive oil. Eur J Clin Invest. 2005 Jul;35(7):421-4.
David
On April 20, a study in BMC Genomics was published that found that olive oil eaten at breakfast modified gene expression in patients with metabolic syndrome (1). The breakfast caused the changes in mononuclear cells after intake of the olive oil and repressed pro-inflammatory genes (1).
The study was performed on 20 patients in a double-blind randomized trial (1). The researchers noted that many of the genes were also implicated in type 2 diabetes, dyslipidemia and obesity (1).
The study adds to evidence that olive oil helps reduce inflammation unlike other oils such as butter (2) and, thereby, adds to the reasons why the Mediterranean diet is associated with lower risk of cardiovascular disease (3).
References
1. Camargo A, Ruano J, Fernandez JM, Parnell LD, Jimenez A, Santos-Gonzalez M, Marin C, Perez-Martinez P, Uceda M, Lopez-Miranda J, Perez-Jimenez F. Gene expression changes in mononuclear cells from patients with metabolic syndrome after acute intake of phenol-rich virgin olive oil. BMC Genomics. 2010 Apr 20;11(1):253. [Epub ahead of print]
2. Bellido C, López-Miranda J, Blanco-Colio LM, Pérez-Martínez P, Muriana FJ, Martín-Ventura JL, Marín C, Gómez P, Fuentes F, Egido J, Pérez-Jiménez F. Butter and walnuts, but not olive oil, elicit postprandial activation of nuclear transcription factor kappaB in peripheral blood mononuclear cells from healthy men.Am J Clin Nutr. 2004 Dec;80(6):1487-91.
3. Bellido C. Perez-Jimenez F, Alvarez de Cienfuegos G, Badimon L, Barja G, Battino M, Blanco A, Bonanome A, Colomer R, Corella-Piquer D, Covas I, Chamorro-Quiros J, Escrich E, Gaforio JJ, Garcia Luna PP, Hidalgo L, Kafatos A, Kris-Etherton PM, Lairon D, Lamuela-Raventos R, Lopez-Miranda J, Lopez-Segura F, Martinez-Gonzalez MA, Mata P, Mataix J, Ordovas J, Osada J, Pacheco-Reyes R, Perucho M, Pineda-Priego M, Quiles JL, Ramirez-Tortosa MC, Ruiz-Gutierrez V, Sanchez-Rovira P, Solfrizzi V, Soriguer-Escofet F, de la Torre-Fornell R, Trichopoulos A, Villalba-Montoro JM, Villar-Ortiz JR, Visioli F. International conference on the healthy effect of virgin olive oil. Eur J Clin Invest. 2005 Jul;35(7):421-4.
David
Labels:
nutr therap
Long distance running causes heart disease, unless it doesn’t
Regardless of type of exercise, disease markers are generally associated with intensity of exertion over time. This association follows a J-curve pattern. Do too little of it, and you have more disease; do too much, and incidence of disease goes up. There is always an optimal point, for each type of exercise and marker. A J curve is actually a U curve, with a shortened left end. The reason for the shortened left end is that, when measurements are taken, usually more measures fall on the right side of the curve than on the left.
The figure below (click to enlarge) shows a schematic representation that illustrates this type of relationship. (I am not very good at drawing.) Different individuals have different curves. If the vertical axis was a measure of health, as opposed to disease, then the curve would have the shape of an inverted J.
The idea that long distance running causes heart disease has been around for a while. Is it correct?
If it is, then one would expect to see certain things. For example, let’s say you take a group of long distance runners who have been doing that for a while, ideally runners above age 50. That is when heart disease becomes more frequent. This would also capture more experienced runners, with enough running experience to cause some serious damage. Let us say you measured markers of heart disease before and after a grueling long distance race. What would you see?
If long distance running causes heart disease, you would see a significant proportion with elevated makers of heart disease among the runners at baseline (i.e., before the race). After all, running is causing a cumulative problem. The levels of those markers would be correlated with practice, or participation in previous races, since the races are causing the damage. Also, you would see a uniformly bad increase in the markers after the race, as the running is messing up everybody more or less equally.
Sahlén and colleagues (2009), a group of Swedish researchers, studied males and females aged 55 or older who participated in a 30-km (about 19-mile) cross-country race. The full reference to the article is at the end of this post. The researchers included only runners who had no diagnosed medical disorders in their study. They collected data on the patterns of exercise prior to the race, and participation in previous races. Blood was taken before and after the race, and several measurements were obtained, including measurements of two possible heart disease markers: N-terminal pro-brain natriuretic peptide (NT-proBNP), and troponin T (TnT). The table below (click to enlarge) shows several of those measurements before and after the race.
We can see that NT-proBNP and TnT increased significantly after the race. So did creatinine, a byproduct of breakdown in muscle tissue of creatine phosphate; something that you would expect after such a grueling race. Yep, long distance running increases NT-proBNP and TnT, so it leads to heart disease, right?
Wait, not so fast!
NT-proBNP and TnT levels usually increase after endurance exercise, something that is noted by the authors in their literature review. But those levels do not stay elevated for too long after the race. Being permanently elevated, that is a sign of a problem. Also, excessive elevation during the race is also a sign of a potential problem.
Now, here is something interesting. Look at the table below, showing the variations grouped by past participation in races.
The increases in NT-proBNP and TnT are generally lower in those individuals that participated in 3 to 13 races in the past. They are higher for the inexperienced runners, and, in the case of NT-proBNP, particularly for those with 14 or more races under their belt (the last group on the right). The baseline NT-proBNP is also significantly higher for that group. They were older too, but not by much.
Can you see a possible J-curve pattern?
Now look at this table below, which shows the results of a multiple regression analysis on its right side. Look at the last column on the right, the beta coefficients. They are all significant, but the first is .81, which is quite high for a standardized partial regression coefficient. It refers to an almost perfect relationship between the log of NT-proBNP increase and the log of baseline NT-proBNP. (The log transformations reflect the nonlinear relationships between NT-proBNP, a fairly sensitive health marker, and the other variables.)
In a multiple regression analysis, the effect of each independent variable (i.e., each predictor) on the dependent variable (the log of NT-proBNP increase) is calculated controlling for the effects of all the other independent variables on the dependent variable. Thus, what the table above is telling us is that baseline NT-proBNP predicts NT-proBNP increase almost perfectly, even when we control for age, creatinine increase, and race duration (i.e., amount of time a person takes to complete the race).
Again, even when we control for: AGE, creatinine increase, and RACE DURATION.
In order words, baseline NT-proBNP is what really matters; not even age makes that much of a difference. But baseline NT-proBNP is NEGATIVELY correlated with number of previous races. The only exception is the group that participated in 14 or more previous races. Maybe that was too much for them.
Okay, one more table. This one, included below, shows regression analyses between a few predictors and the main dependent variable, which in this case is TnT elevation. No surprises here based on the discussion so far. Look at the left part, the column labeled as “B”. Those are correlation coefficients, varying from -1 to 1. Which is the predictor with the highest absolute correlation with TnT elevation? It is number of previous races, but the correlation is, again, NEGATIVE.
In follow-up tests after the race, 9 out of the 185 participants (4.9 percent) showed more decisive evidence of heart disease. One of those died while training a few months after the race. An autopsy was conducted showing abnormal left ventricular hypertrophy with myocardial fibrosis, coronary artery narrowing, and an old myocardial scar.
Who were the 9 lucky ones? You guessed it. Those were the ones who had the largest increases in NT-proBNP during the race. And large increases in NT-proBNP were more common among the runners who were too inexperienced or too experienced. The ones at the extremes.
So, here is a summary of what this study is telling us:
- The 30-km cross-country race studied is no doubt a strenuous activity. So if you have not exercised in years, perhaps you should not start with this kind of race.
- By and large, individuals who had elevated markers of heart disease prior to the race also had the highest elevations of those markers after the race.
- Participation in past races was generally protective, likely due to compensatory body adaptations, with the exception of those who did too much of that.
- Prevalence of heart disease among the runners was measured at 4.9 percent. This does not beat even the mildly westernized Inuit, but certainly does not look so bad considering that the general prevalence of ischemic heart disease in the US and Sweden is about 6.8 percent.
It seems reasonable to conclude that long distance running may be healthy, unless one does too much of it. The ubiquitous J-curve pattern again.
How much is too much? It certainly depends on each person’s particular health condition, but the bar seems to be somewhat high on average: participation in 14 or more previous 30-km races.
As for the 4.9 percent prevalence of heart disease among runners, maybe it is caused by something else, and endurance running may actually be protective, as long as it is not taken to extremes. Maybe that something else is a diet rich in refined carbohydrates and sugars, or psychological stress caused by modern life, or a combination of both.
Just for the record, I don’t do endurance running. I like walking, sprinting, moderate resistance training, and also a variety of light aerobic activities that involve some play. This is just a personal choice; nothing against endurance running.
Mark Sisson was an accomplished endurance runner; now he does not like it very much. (Click here to check his excellent book The Primal Blueprint). Arthur De Vany is not a big fan of endurance running either.
Still, maybe the Tarahumara and hunter-gatherer groups who practice persistence hunting are not such huge exceptions among humans after all.
Reference:
Sahlén, A., Gustafsson, T.P., Svensson, J.E., Marklund, T., Winter, R., Linde, C., & Braunschweig, F. (2009). Predisposing Factors and Consequences of Elevated Biomarker Levels in Long-Distance Runners Aged >55 Years. The American Journal of Cardiology, 104(10), 1434–1440.
The figure below (click to enlarge) shows a schematic representation that illustrates this type of relationship. (I am not very good at drawing.) Different individuals have different curves. If the vertical axis was a measure of health, as opposed to disease, then the curve would have the shape of an inverted J.
The idea that long distance running causes heart disease has been around for a while. Is it correct?
If it is, then one would expect to see certain things. For example, let’s say you take a group of long distance runners who have been doing that for a while, ideally runners above age 50. That is when heart disease becomes more frequent. This would also capture more experienced runners, with enough running experience to cause some serious damage. Let us say you measured markers of heart disease before and after a grueling long distance race. What would you see?
If long distance running causes heart disease, you would see a significant proportion with elevated makers of heart disease among the runners at baseline (i.e., before the race). After all, running is causing a cumulative problem. The levels of those markers would be correlated with practice, or participation in previous races, since the races are causing the damage. Also, you would see a uniformly bad increase in the markers after the race, as the running is messing up everybody more or less equally.
Sahlén and colleagues (2009), a group of Swedish researchers, studied males and females aged 55 or older who participated in a 30-km (about 19-mile) cross-country race. The full reference to the article is at the end of this post. The researchers included only runners who had no diagnosed medical disorders in their study. They collected data on the patterns of exercise prior to the race, and participation in previous races. Blood was taken before and after the race, and several measurements were obtained, including measurements of two possible heart disease markers: N-terminal pro-brain natriuretic peptide (NT-proBNP), and troponin T (TnT). The table below (click to enlarge) shows several of those measurements before and after the race.
We can see that NT-proBNP and TnT increased significantly after the race. So did creatinine, a byproduct of breakdown in muscle tissue of creatine phosphate; something that you would expect after such a grueling race. Yep, long distance running increases NT-proBNP and TnT, so it leads to heart disease, right?
Wait, not so fast!
NT-proBNP and TnT levels usually increase after endurance exercise, something that is noted by the authors in their literature review. But those levels do not stay elevated for too long after the race. Being permanently elevated, that is a sign of a problem. Also, excessive elevation during the race is also a sign of a potential problem.
Now, here is something interesting. Look at the table below, showing the variations grouped by past participation in races.
The increases in NT-proBNP and TnT are generally lower in those individuals that participated in 3 to 13 races in the past. They are higher for the inexperienced runners, and, in the case of NT-proBNP, particularly for those with 14 or more races under their belt (the last group on the right). The baseline NT-proBNP is also significantly higher for that group. They were older too, but not by much.
Can you see a possible J-curve pattern?
Now look at this table below, which shows the results of a multiple regression analysis on its right side. Look at the last column on the right, the beta coefficients. They are all significant, but the first is .81, which is quite high for a standardized partial regression coefficient. It refers to an almost perfect relationship between the log of NT-proBNP increase and the log of baseline NT-proBNP. (The log transformations reflect the nonlinear relationships between NT-proBNP, a fairly sensitive health marker, and the other variables.)
In a multiple regression analysis, the effect of each independent variable (i.e., each predictor) on the dependent variable (the log of NT-proBNP increase) is calculated controlling for the effects of all the other independent variables on the dependent variable. Thus, what the table above is telling us is that baseline NT-proBNP predicts NT-proBNP increase almost perfectly, even when we control for age, creatinine increase, and race duration (i.e., amount of time a person takes to complete the race).
Again, even when we control for: AGE, creatinine increase, and RACE DURATION.
In order words, baseline NT-proBNP is what really matters; not even age makes that much of a difference. But baseline NT-proBNP is NEGATIVELY correlated with number of previous races. The only exception is the group that participated in 14 or more previous races. Maybe that was too much for them.
Okay, one more table. This one, included below, shows regression analyses between a few predictors and the main dependent variable, which in this case is TnT elevation. No surprises here based on the discussion so far. Look at the left part, the column labeled as “B”. Those are correlation coefficients, varying from -1 to 1. Which is the predictor with the highest absolute correlation with TnT elevation? It is number of previous races, but the correlation is, again, NEGATIVE.
In follow-up tests after the race, 9 out of the 185 participants (4.9 percent) showed more decisive evidence of heart disease. One of those died while training a few months after the race. An autopsy was conducted showing abnormal left ventricular hypertrophy with myocardial fibrosis, coronary artery narrowing, and an old myocardial scar.
Who were the 9 lucky ones? You guessed it. Those were the ones who had the largest increases in NT-proBNP during the race. And large increases in NT-proBNP were more common among the runners who were too inexperienced or too experienced. The ones at the extremes.
So, here is a summary of what this study is telling us:
- The 30-km cross-country race studied is no doubt a strenuous activity. So if you have not exercised in years, perhaps you should not start with this kind of race.
- By and large, individuals who had elevated markers of heart disease prior to the race also had the highest elevations of those markers after the race.
- Participation in past races was generally protective, likely due to compensatory body adaptations, with the exception of those who did too much of that.
- Prevalence of heart disease among the runners was measured at 4.9 percent. This does not beat even the mildly westernized Inuit, but certainly does not look so bad considering that the general prevalence of ischemic heart disease in the US and Sweden is about 6.8 percent.
It seems reasonable to conclude that long distance running may be healthy, unless one does too much of it. The ubiquitous J-curve pattern again.
How much is too much? It certainly depends on each person’s particular health condition, but the bar seems to be somewhat high on average: participation in 14 or more previous 30-km races.
As for the 4.9 percent prevalence of heart disease among runners, maybe it is caused by something else, and endurance running may actually be protective, as long as it is not taken to extremes. Maybe that something else is a diet rich in refined carbohydrates and sugars, or psychological stress caused by modern life, or a combination of both.
Just for the record, I don’t do endurance running. I like walking, sprinting, moderate resistance training, and also a variety of light aerobic activities that involve some play. This is just a personal choice; nothing against endurance running.
Mark Sisson was an accomplished endurance runner; now he does not like it very much. (Click here to check his excellent book The Primal Blueprint). Arthur De Vany is not a big fan of endurance running either.
Still, maybe the Tarahumara and hunter-gatherer groups who practice persistence hunting are not such huge exceptions among humans after all.
Reference:
Sahlén, A., Gustafsson, T.P., Svensson, J.E., Marklund, T., Winter, R., Linde, C., & Braunschweig, F. (2009). Predisposing Factors and Consequences of Elevated Biomarker Levels in Long-Distance Runners Aged >55 Years. The American Journal of Cardiology, 104(10), 1434–1440.
Subscribe to:
Posts (Atom)